Question
Freezing points of solutions: Sample 1: C; AT:= C; Mm = Samples 1 + 2: C; AT= C; Mm !! 3. Freezing points of
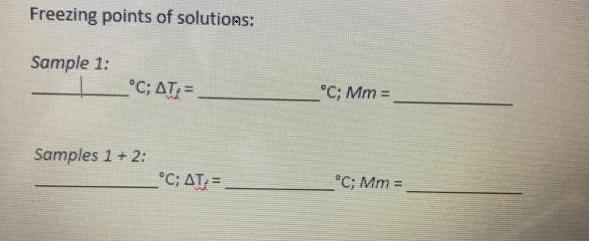
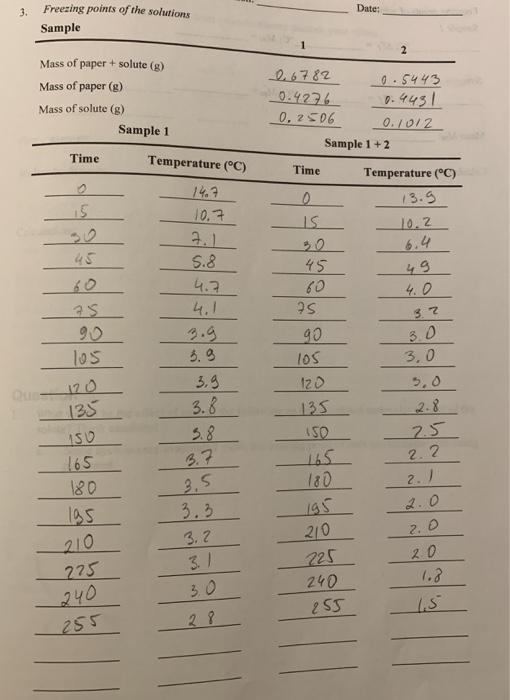
Freezing points of solutions: Sample 1: C; AT:= C; Mm = Samples 1 + 2: C; AT= C; Mm !! 3. Freezing points of the solutions Date: Sample Mass of paper + solute (g) 2 26782 0.4276 0, 2506 Mass of paper (g) 0.5443 Mass of solute (g) 0. 4431 Sample 1 0.1012 Sample 1 +2 Time Temperature (C) Time Temperature (C) 14.7 13.5 10.7 10.2 30 6.4 49 4.0 45 5.8 4.7 4.1 45 60 90 3.9 3 90 3.0 3,0 l05 l05 3.3 3.8 Que 120 120 5,0 125 135 2.8 5.8 3.7 3.5 3.3 150 SO 7.5 2.2 2.) 2.0 165 180 180 195 195 210 3.2 3.1 2. 0 0 (.3 210 225 240 255 275 240 255 30 1.5
Step by Step Solution
3.27 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Y Freezing Points of Solutions IA Sample 1 C ATE C Mm To calculate the freeing Point o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



