

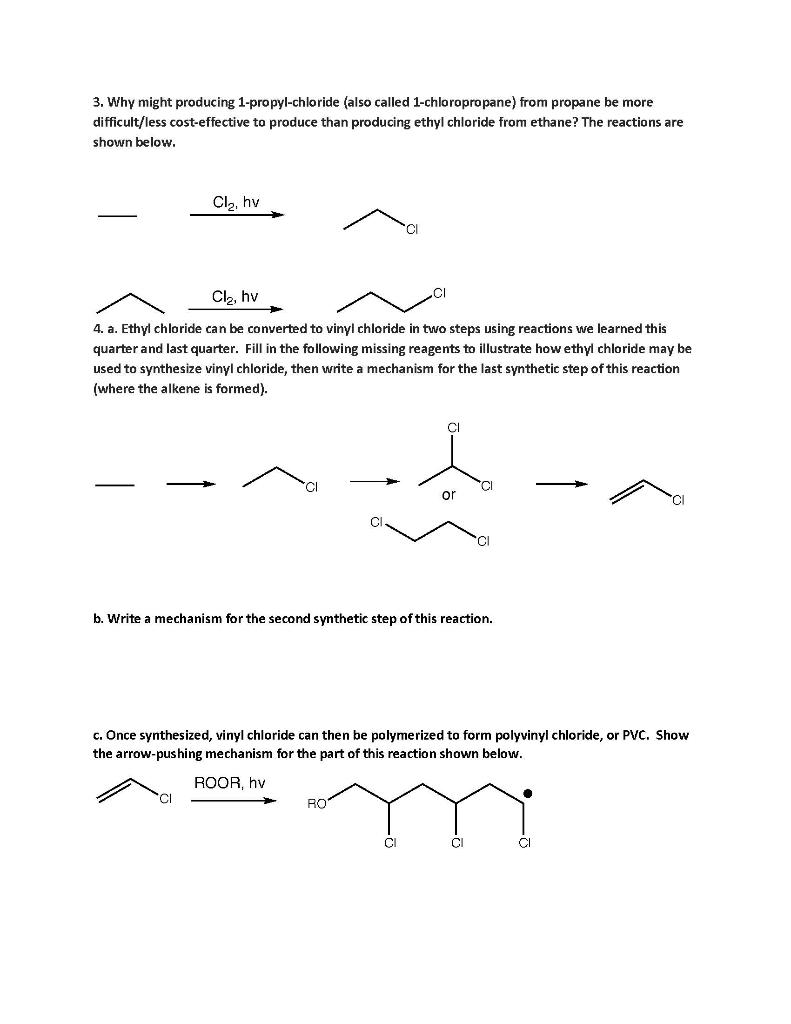
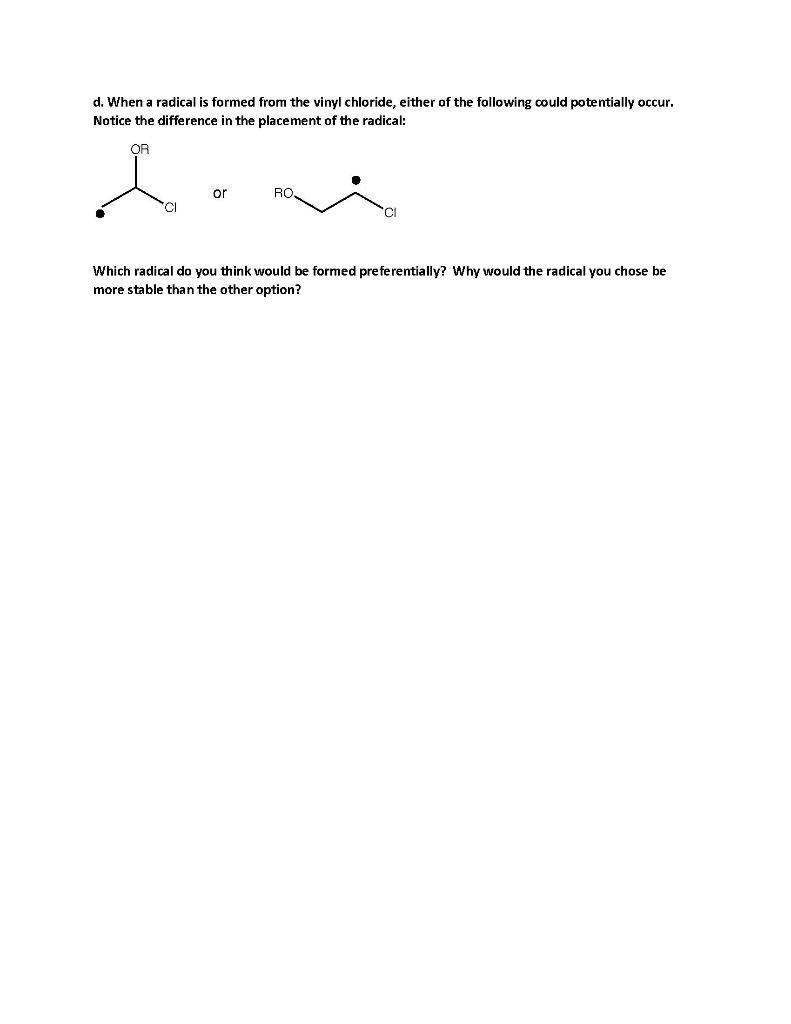
Freezing Teeth "MOO00000OM!" Camie knew it was more than a bad dream when her daughter screamed like that. "I'm coming little one" she said, trying (and failing) to sound soothing as she bolted out of bed and ran to Anna's room. "What is it?" "My mouf hurts." Anna clutched her cheek. "Let me see Anna" said Camie, gently pulling Anna's little hand away from her swollen cheek. She pulled out a flashlight and looked inside Anna's mouth. "Well little one, it's probably a toothache. Remember how a few weeks ago you fell down and hit your tooth on the floor?" "Yeah, I cried a lot" Anna remembered, sniffling. "Well, that tooth has turned dark, which probably means it's dead or dying." Camie worked as a pediatric dental hygienist and saw this fairly frequently, though she was embarrassed to miss it in her own daughter. "My toof is DEAD?!" Anna panicked. She had recently become acquainted with death as her hamster, Sir Fluffernutter Whiskers, passed away last year. "Not like Fluffernutter sweetie, it just means that the blood flow has been cut off. Luckily that's a baby tooth and will fall out soon anyway, but we will have to go to the dentist tomorrow." "I'm going to work wif you?" squealed Anna, momentarily forgetting both the sorrow of losing Fluffernutter and her tooth pain. "Yep. It's my day off but I know that we're not very busy tomorrow, so I'm sure Dr. Johnson can squeeze you in." "And my mouf won't hurt anymore?" "Right-o kiddo! Now stay here and I'll get you a cold pack of ice and some medicine that will help you feel better." "Mom, what's that?" Anna pointed to a canister at the dentist's office. Camie had called Dr. Johnson that morning before work and he agreed to see Anna right when they opened. She couldn't assist as it was her daughter, but she asked to be in the room with Anna to comfort her. "That is a special spray Anna, and Dr. Johnson is going to spray it on some cotton and then put it on your tooth." "Like hairspray?" "Well, not quite. This is a special spray that feels really cold- in fact it's called 'Endo Ice.' Dr. Johnson is going to use it to tell if your tooth is dead." "Oh, okay. If it's dead, do you think my tooth will say hi to Fluffernutter and tell him I miss playing with him?" "I'm sure Fluffernutter knows. Now I want you to be brave. This might hurt a teensy bit. As soon as your tooth is fixed, we'll go to the grocery store and you can pick out your very favorite ice cream." "Okay Mom." HYCENIC ENDO I.C.E REFRIGERANT SPRAY - COLTENE FOR THE DETECTION OF PULP VITALITY The Endolce that Camie referred to is a refrigerant spray used in dentistry to test for a dead tooth. It has a low boiling point which causes it to boil rapidly at room temperature. In doing so, it absorbs energy from the tooth and causes a freezing sensation which is painful if the nerve inside the tooth is still intact; however, if the patient experiences no pain, the nerve has died and the tooth must either be extracted or undergo a root canal. MALLORCA 1. Endo ice is a fancy name for a chemical familiar to us: ethyl chloride (also called chloroethane, CH3CH2CI). a. Why do smaller molecules tend to have lower boiling points than larger molecules? ("Because they're smaller" is not an appropriate answer. I'm looking to see that you understand why rather than just memorizing a trend.) b. Methanol (CH3OH) is smaller than ethyl chloride; however, it has a significantly higher boiling point. It is liquid at room temperature, whereas ethyl chloride is gaseous at room temperature. Why? 2. Ethyl chloride is not naturally occurring and thus must be produced industrially. One way of accomplishing this is through the radical halogenation of ethane. Write the arrow.pushing mechanism for this reaction and label the chain initiating, chain propagating, and chain terminating steps. 3. Why might producing 1-propyl-chloride (also called 1-chloropropane) from propane be more difficult/less cost-effective to produce than producing ethyl chloride from ethane? The reactions are shown below. Cla, hv CI Cl, hv 4. a. Ethyl chloride can be converted to vinyl chloride in two steps using reactions we learned this quarter and last quarter. Fill in the following missing reagents to illustrate how ethyl chloride may be used to synthesize vinyl chloride, then write a mechanism for the last synthetic step of this reaction (where the alkene is formed). CI or b. Write a mechanism for the second synthetic step of this reaction. c. Once synthesized, vinyl chloride can then be polymerized to form polyvinyl chloride, or PVC. Show the arrow-pushing mechanism for the part of this reaction shown below. ROOR, hv CI RO CI CI d. When a radical is formed from the vinyl chloride, either of the following could potentially occur. Notice the difference in the placement of the radical: OR or RO 0 CI Which radical do you think would be formed preferentially? Why would the radical you chose be more stable than the other option










