Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemical engineer is studying the rate of this reaction. 2SO3 (g) 2SO(g) + O(g) 2 He fills a reaction vessel with SO3 and
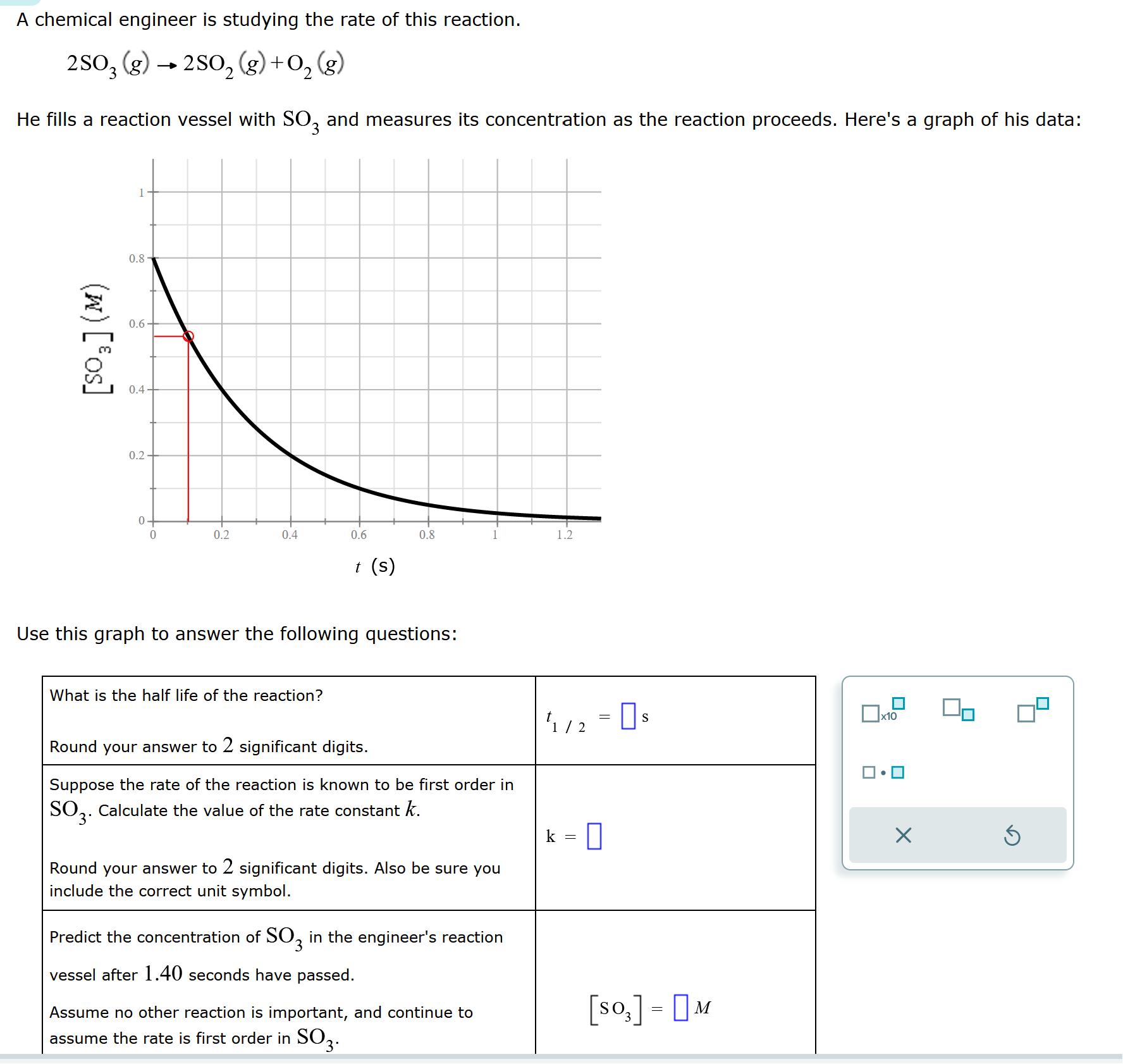
A chemical engineer is studying the rate of this reaction. 2SO3 (g) 2SO(g) + O(g) 2 He fills a reaction vessel with SO3 and measures its concentration as the reaction proceeds. Here's a graph of his data: (w) [os] 0.8 0.6- 0.4 0.2 0+ 0 O 0.2 0.4 0.6 What is the half life of the reaction? t (s) 0.8 Use this graph to answer the following questions: Round your answer to 2 significant digits. Suppose the rate of the reaction is known to be first order in SO3. Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure you include the correct unit symbol. Predict the concentration of SO3 in the engineer's reaction vessel after 1.40 seconds have passed. Assume no other reaction is important, and continue to assume the rate is first order in SO3. 1.2 1/2 = 0 s k 0 [SO] - M = x10 X 5
Step by Step Solution
★★★★★
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
First lets define some key terms 1 Vmax is the maximum rate of an enzymecatalyzed reaction 2 kcat is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


