Answered step by step
Verified Expert Solution
Question
1 Approved Answer
gas with Cp* = (7/2)R. 4-29. Throughout Chapters 3 and 4, we have assumed that potential and kinetic energy are negligible in standard chemical process
gas with Cp* = (7/2)R.
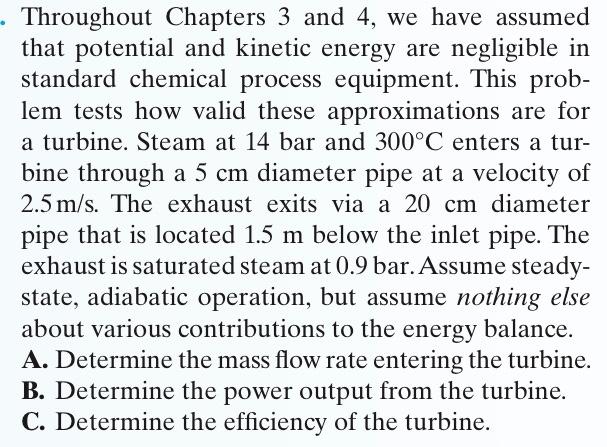
4-29. Throughout Chapters 3 and 4, we have assumed that potential and kinetic energy are negligible in standard chemical process equipment. This prob- lem tests how valid these approximations are for a turbine. Steam at 14 bar and 300C enters a tur- bine through a 5 cm diameter pipe at a velocity of 2.5m/s. The exhaust exits via a 20 cm diameter pipe that is located 1.5 m below the inlet pipe. The exhaust is saturated steam at 0.9 bar.Assume steady- state, adiabatic operation, but assume nothing else about various contributions to the energy balance. A. Determine the mass flow rate entering the turbine. B. Determine the power output from the turbine. C. Determine the efficiency of the turbine.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


