Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreting cells. The cells secrete HCI, proteolytic enzyme zymogens, mucin,
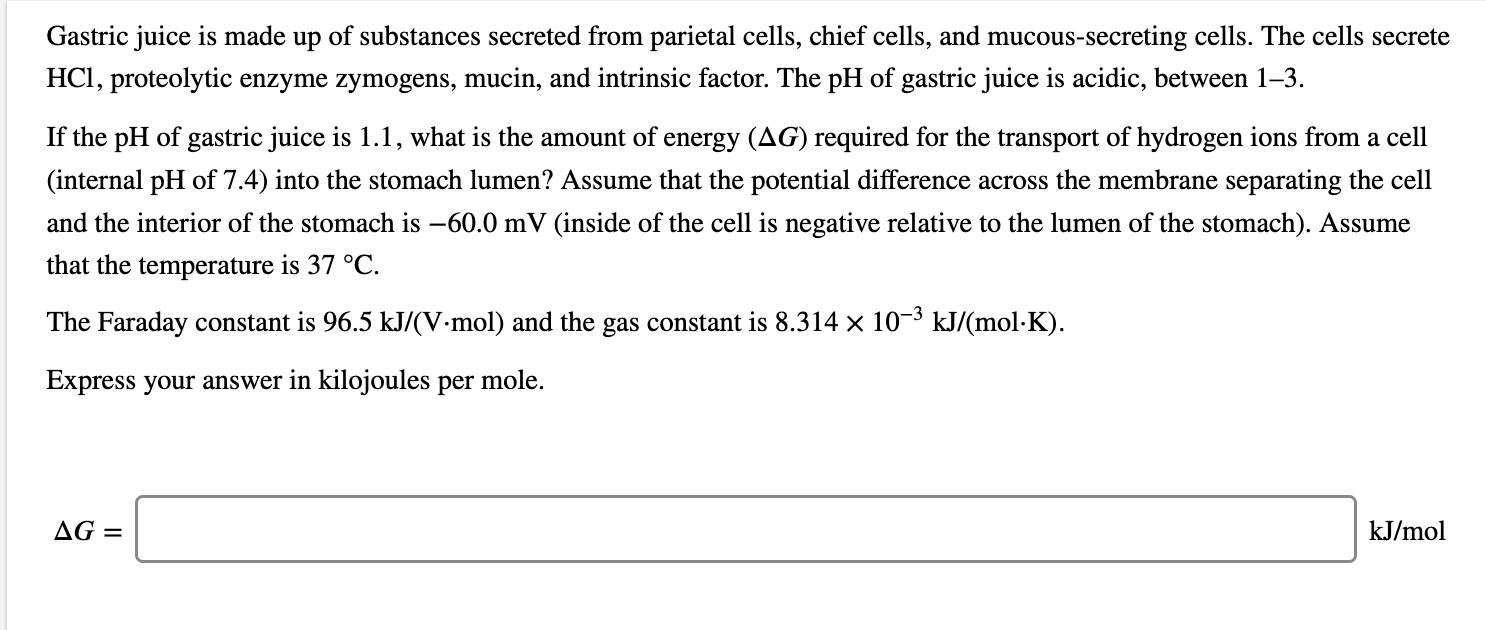
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreting cells. The cells secrete HCI, proteolytic enzyme zymogens, mucin, and intrinsic factor. The pH of gastric juice is acidic, between 1-3. If the pH of gastric juice is 1.1, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the potential difference across the membrane separating the cell and the interior of the stomach is -60.0 mV (inside of the cell is negative relative to the lumen of the stomach). Assume that the temperature is 37 C. The Faraday constant is 96.5 kJ/(V-mol) and the gas constant is 8.314 x 10-3 kJ/(molK). Express your answer in kilojoules per mole. AG = kJ/mol
Step by Step Solution
★★★★★
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Amount of energy required foe transport of hydrogen ions from a cell into stomach lumen is given as ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


