Answered step by step
Verified Expert Solution
Question
1 Approved Answer
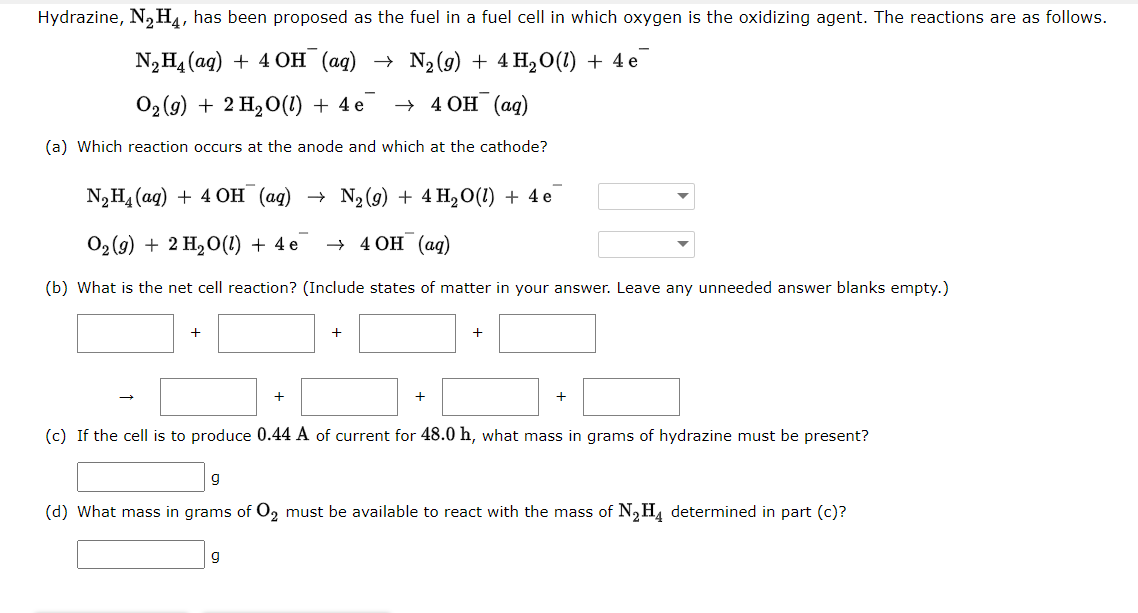
General Chemistry for Engineers Lesson: Electrochemistry Directions: Answer the following problems by showing the complete solution. In return, I will give you a good rating.
General Chemistry for Engineers
Lesson: Electrochemistry
Directions: Answer the following problems by showing the complete solution. In return, I will give you a good rating. Thank you so much!
Note: Please be careful with the units and measurements in your calculations. And also, the question in section "a" is that the arrow down icon has two choices, "canode or anode." Thank you. Thank you so much!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


