Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Give the correct answer for the following questions Given the following data for measuring the hardness of water; the volume of the water was 25.00
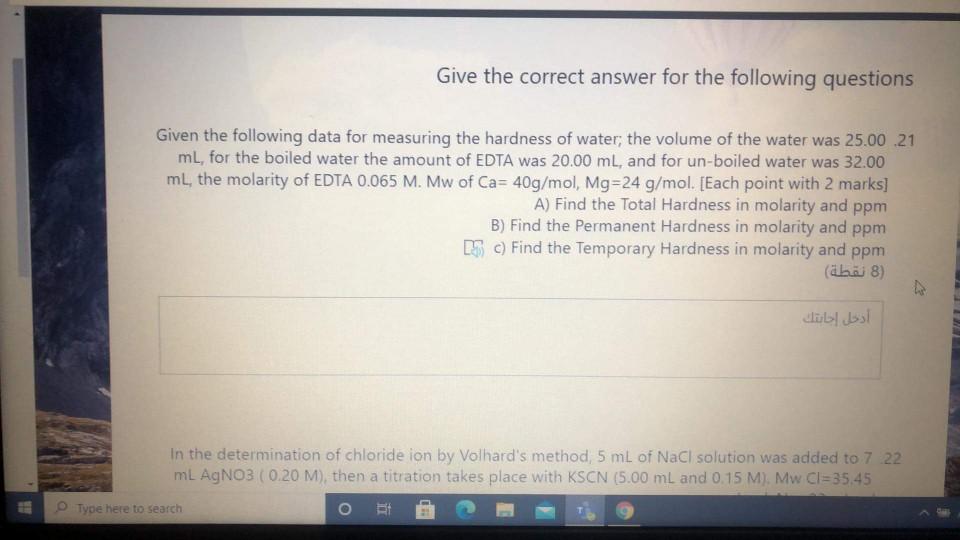
Give the correct answer for the following questions Given the following data for measuring the hardness of water; the volume of the water was 25.00 21 ml, for the boiled water the amount of EDTA was 20.00 mL, and for un-boiled water was 32.00 ml, the molarity of EDTA 0.065 M. Mw of Ca= 40g/mol, Mg=24 g/mol. [Each point with 2 marks] A) Find the Total Hardness in molarity and ppm B) Find the Permanent Hardness in molarity and ppm LS C) Find the Temporary Hardness in molarity and ppm () (8 ) MIT In the determination of chloride ion by Volhard's method 5 mL of NaCl solution was added to 7 .22 ml AgNO3 (0.20 M), then a titration takes place with KSCN (5.00 mL and 0.15 M). Mw CI=35.45 . Type here to search
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


