Answered step by step
Verified Expert Solution
Question
1 Approved Answer
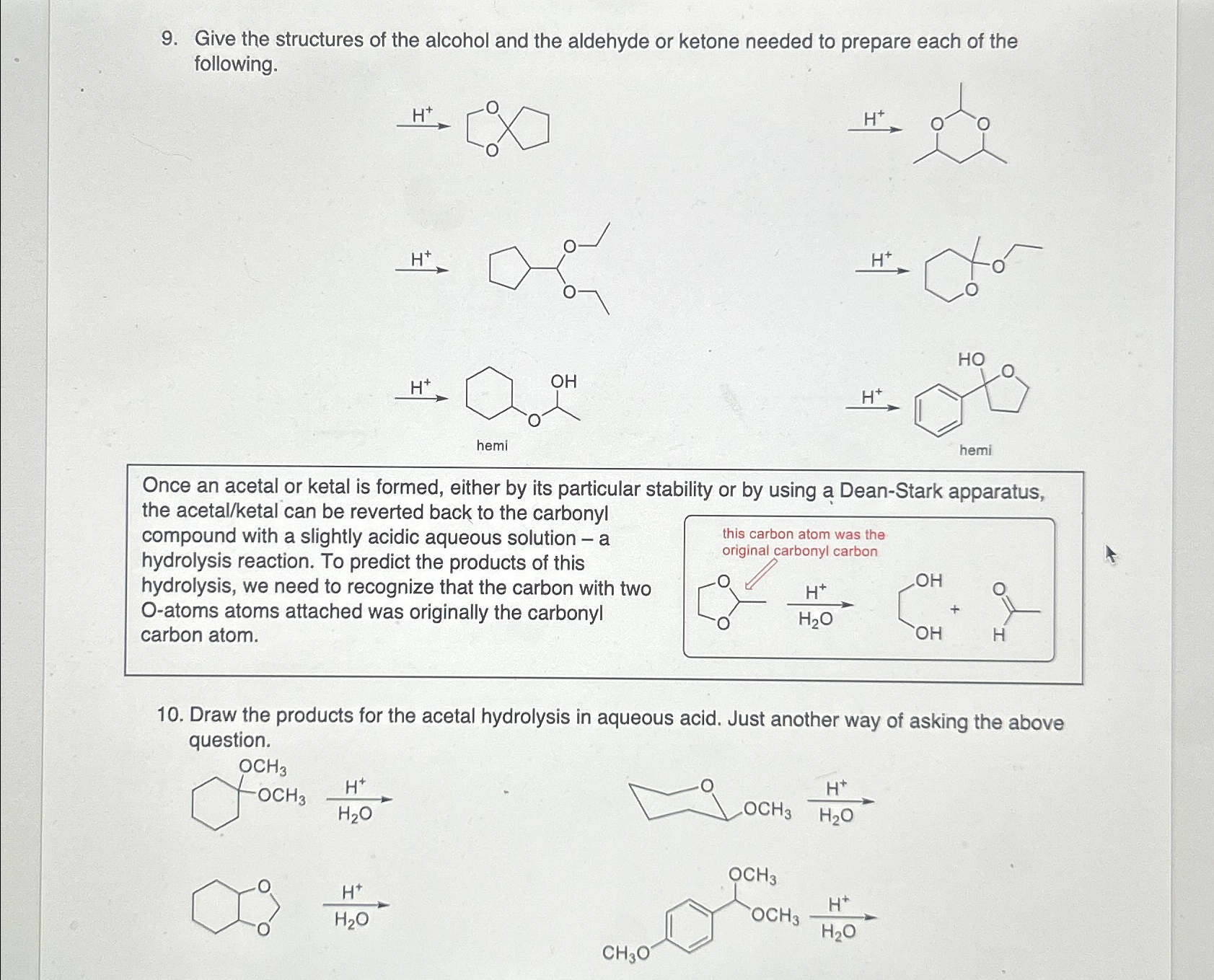
Give the structures of the alcohol and the aldehyde or ketone needed to prepare each of the following. Once an acetal or ketal is formed,
Give the structures of the alcohol and the aldehyde or ketone needed to prepare each of the following.
Once an acetal or ketal is formed, either by its particular stability or by using a DeanStark apparatus, the acetalketal can be reverted back to the carbonyl compound with a slightly acidic aqueous solution a hydrolysis reaction. To predict the products of this hydrolysis, we need to recognize that the carbon with two Oatoms atoms attached was originally the carbonyl carbon atom.
Draw the products for the acetal hydrolysis in aqueous acid. Just another way of asking the above question.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


