Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A catalytic chemical reaction given in (A3.1) that was run at 3.5 atm and 120C in a PER unit that contained 10ce catalys and
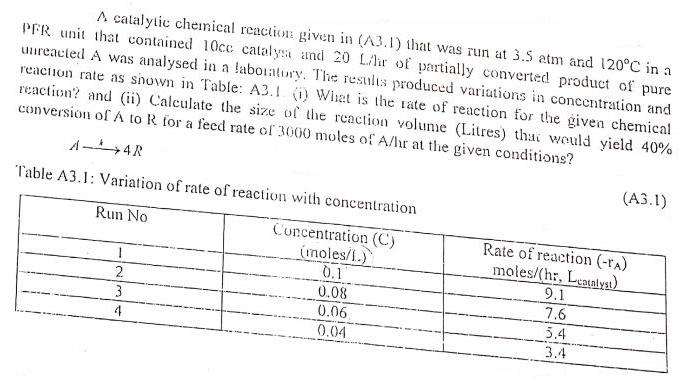
A catalytic chemical reaction given in (A3.1) that was run at 3.5 atm and 120C in a PER unit that contained 10ce catalys and 20 L/lir of partially converted product of pure uureacted A was analysed in a laboratory. The results produced variations in concentration and reaction rate as shown in Table: A3.1 () Whet is the rate of reaction for the given chemical reaction? and (ii) Calculate the size of the rcaction volume (Litres) thai weuld yield 40% conversion of A to R for a feed rate of 3000 moles of A/lhr at the given conditions? A-4R (.1) Table A3.1: Variation of rate of reaction with concentration Run No Concentration (C) (noles/1.) 0.1 Rate of reaction (-rA) moles/(hr. Leatalys) 2. 3 0.08 0.06 9.1 7.6 5.4 4 0.04 3.4
Step by Step Solution
★★★★★
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
A catalytic chemical reaction PFR reactor The plug floe reactor model is a model used to describes chemical reactions in continuous flowing system of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


