Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given a reaction that occurs through the collision of A and B. Calculate the rate law, and show when it behaves as a diffusion-controlled
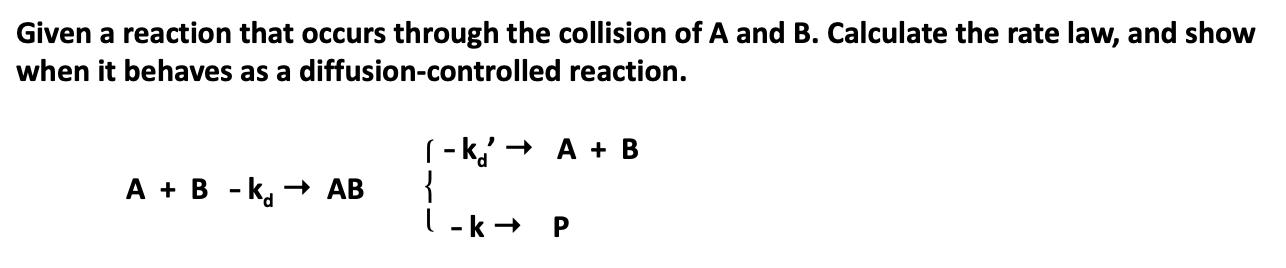
Given a reaction that occurs through the collision of A and B. Calculate the rate law, and show when it behaves as a diffusion-controlled reaction. (- k,' + A + B { l-k + P A + B - k, AB
Step by Step Solution
★★★★★
3.50 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Collision theory states that when suitable particles of the reactant hit each other only a certain amount of collisions result in a perceptible or not...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6366b10e67706_241024.pdf
180 KBs PDF File
6366b10e67706_241024.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


