Question
Given that the half-reaction on the positive electrode (i.e., the anodic reaction since the battery acts as an electrolytic cell during charging) has a potential
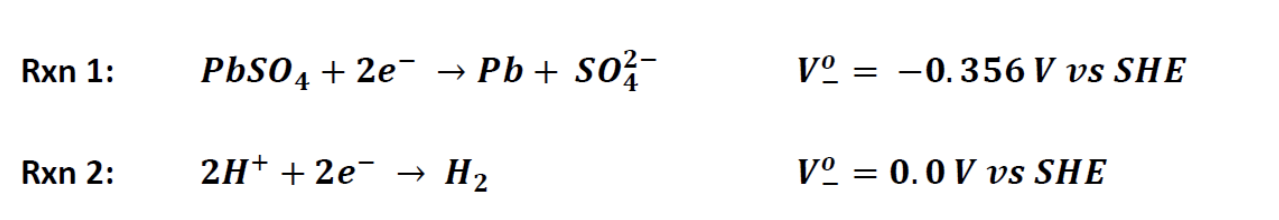
Given that the half-reaction on the positive electrode (i.e., the anodic reaction since the battery acts as an electrolytic cell during charging) has a potential of +1.685 V, both reactions can serve as the other (i.e., cathodic) half-reaction. This problem will explore how the kinetics of the reactions can affect the product distribution.
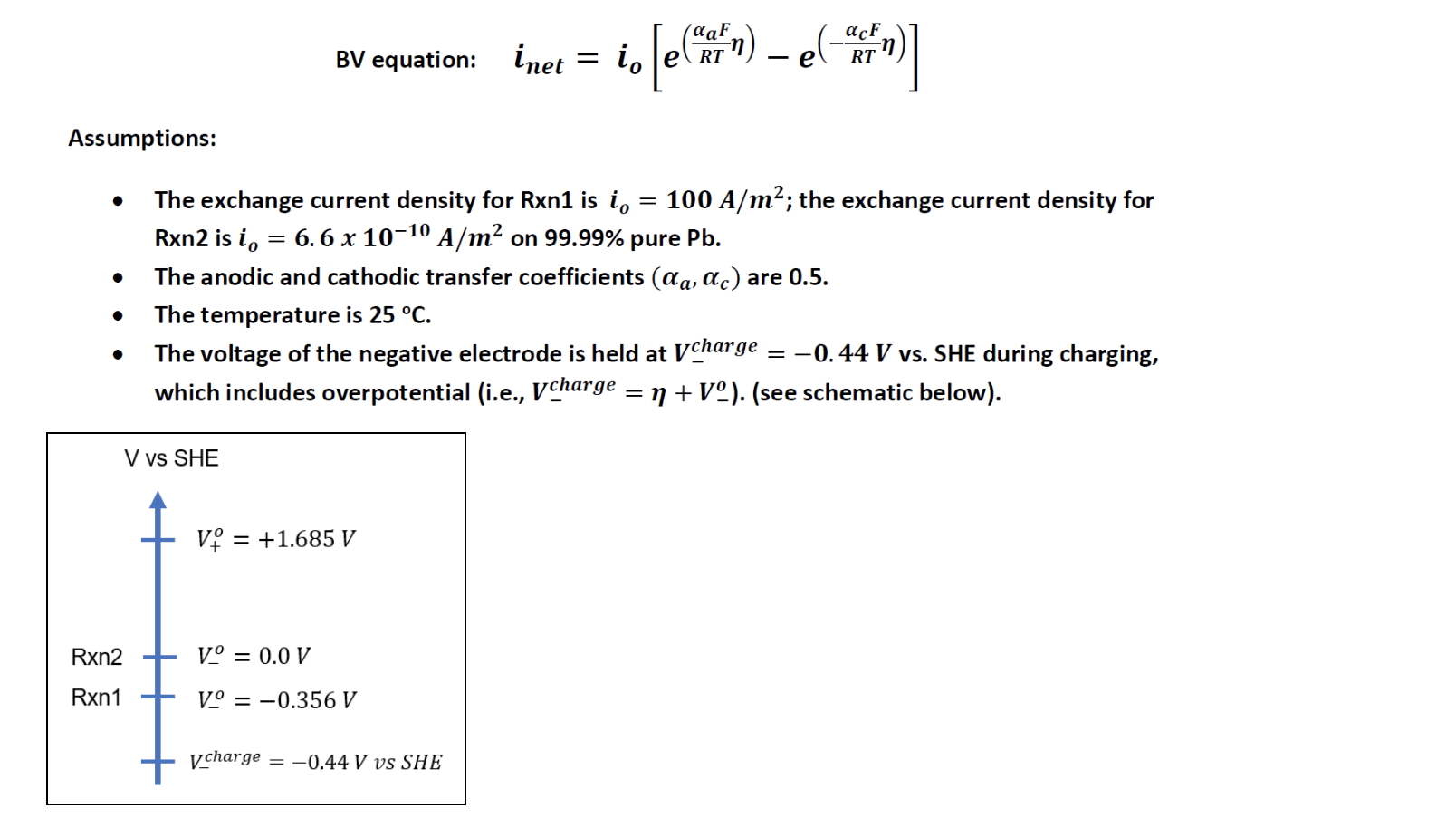
(a) Use the simplified form of the Butler-Volmer (BV) equation below and the following assumptions to calculate the amount of current density expected to be associated with Rxn1 and Rxn2 separately (i.e., use the BV equation for Rxn1 and then use it for Rxn2).
(b) Since pure Pb is a very soft metal, it is usually hardened by adding 2-6 wt% antimony (Sb). The exchange current density for Rxn2 when it takes place on these Pb-Sb alloys is. Repeat (a) for Rxn2 using this exchange current density to see how much the current density associated with Rxn2 changes.
c) Use Faradays law and the current densities you calculated in the previous parts to determine how many moles of H2 compared Pb are produced on an electrode area of 1 m2 over a duration of 1 hour. Then, use the ideal gas law (PV = nRT) to determine the equivalent volume of H2 in liters. How much more H2 is produced on the Pb-Sb alloy compared to pure Pb?
Rxn 1: PbSO4 + 2e- Pb + sox- V -0.356 V vs SHE Rxn 2: 2H+ + 2e H2 V = 0.0 V vs SHE BV equation: tnet acF . is [ein) - eleven RT Assumptions: . = . The exchange current density for Rxn1 is i. = 100 A/m; the exchange current density for Rxn2 is i, = 6.6 x 10-10 A/m2 on 99.99% pure Pb. The anodic and cathodic transfer coefficients (@a, ac) are 0.5. The temperature is 25 C. The voltage of the negative electrode is held at Vcharge -0.44 V vs. SHE during charging, which includes overpotential (i.e., Vcharge = n + V). (see schematic below). . = = V vs SHE Vi = +1.685 V Rxn2 VO = 0.0 V Rxn1 V = -0.356 V v charge = -0.44 V vs SHE TStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


