Question
Given that the molecular weight of a polystyrene (PS) repeating unit is 104 and the the carbon-carbon distance is 1.54 , calculate the following:
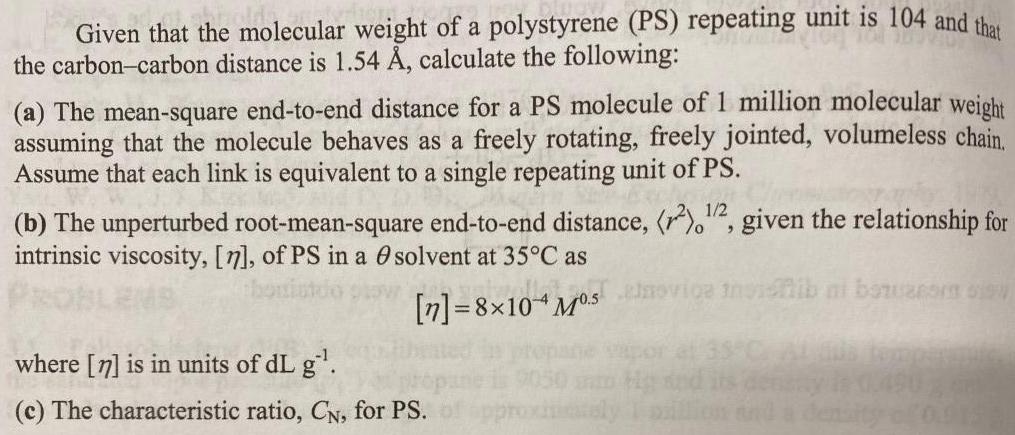
Given that the molecular weight of a polystyrene (PS) repeating unit is 104 and the the carbon-carbon distance is 1.54 , calculate the following: (a) The mean-square end-to-end distance for a PS molecule of 1 million molecular weight assuming that the molecule behaves as a freely rotating, freely jointed, volumeless chain. Assume that each link is equivalent to a single repeating unit of PS. 1/2 (b) The unperturbed root-mean-square end-to-end distance, (r),", given the relationship for intrinsic viscosity, [7], of PS in a O solvent at 35C as [n]=8x10 M5 ovige inidnib ni bawarom o where [n] is in units of dL g. (c) The characteristic ratio, CN, for PS.
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
9th edition
978-1118546895, 111854689X, 978-1118477700, 1118477707, 1118324579, 978-1118324578
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



