Question
Given the atomic mass of select elements, calculate the molar mass of each salt. 28.01 g/mol 43.31 g/mol 47.01 g/mol 82.55 g/mol 62.31 g/mol
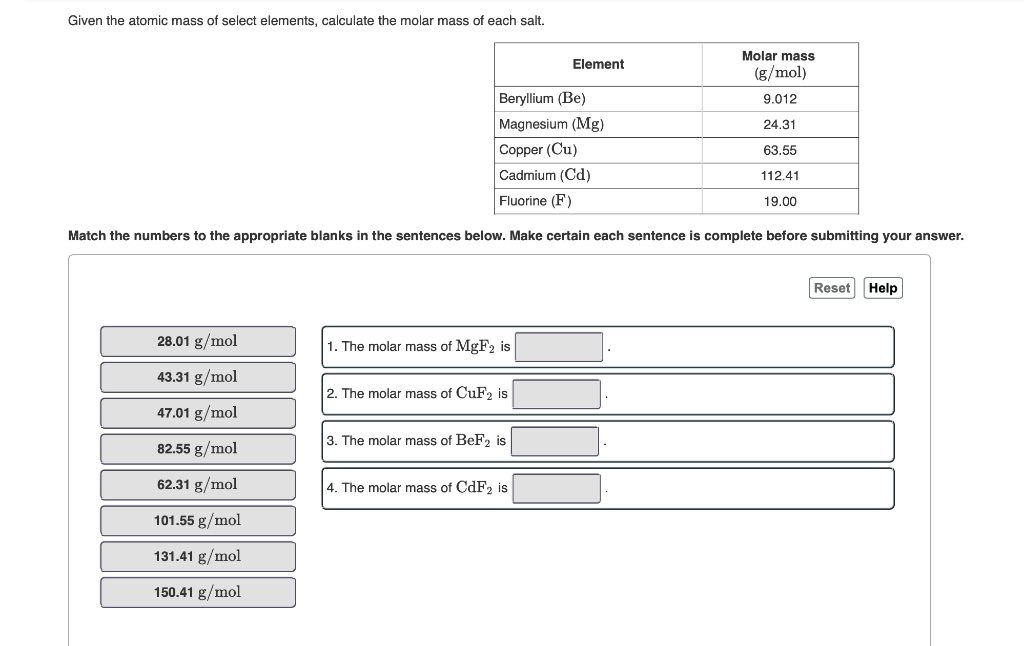
Given the atomic mass of select elements, calculate the molar mass of each salt. 28.01 g/mol 43.31 g/mol 47.01 g/mol 82.55 g/mol 62.31 g/mol 101.55 g/mol 131.41 g/mol 150.41 g/mol Beryllium (Be) Magnesium (Mg) Copper (Cu) Cadmium (Cd) Fluorine (F) Match the numbers to the appropriate blanks in the sentences below. Make certain each sentence is complete before submitting your answer. 1. The molar mass of MgF2 is 2. The molar mass of CuF2 is 3. The molar mass of BeF2 is Element 4. The molar mass of CdF2 is Molar mass (g/mol) 9.012 24.31 63.55 112.41 19.00 Reset Help
Step by Step Solution
3.27 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Given Molur mass of Beryllium m 9012 gmol Be Molar mass of Magnesium MM 2431 9mo 1 molar mas...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics With Modern Physics
Authors: Wolfgang Bauer, Gary Westfall
2nd edition
73513881, 978-0073513881
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



