Question
Given the following data for the following half-reactions and E degree (V vs. NHE): Cu2 + 2e= Cu E=0.340V Cu* + + e=Cul E=0.86V Oz
Given the following data for the following half-reactions and E degree (V vs. NHE):
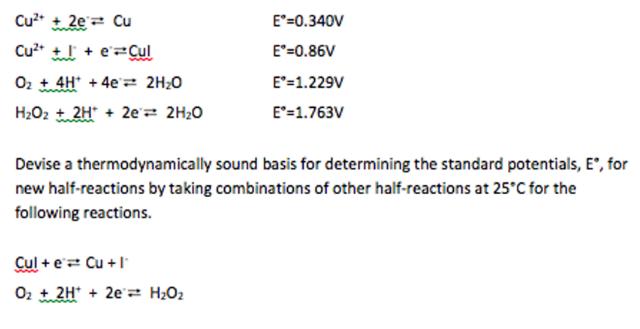
Cu2 + 2e= Cu E=0.340V Cu* + + e=Cul E"=0.86V Oz t4H + 4e 2H;0 E'=1.229V H2O2 t 2H" + 2e'2 2H20 E'=1.763V Devise a thermodynamically sound basis for determining the standard potentials, E', for new half-reactions by taking combinations of other half-reactions at 25C for the following reactions. Cul + e Cu + I Oz + 2H* + 2e= H2O2
Step by Step Solution
There are 3 Steps involved in it
Step: 1
2 1 first we will calculate AG 0 AG nfe cell AG 2FE nno of Le involved 6 AG FE in hal...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
OM operations management
Authors: David Alan Collier, James R. Evans
5th edition
1285451376, 978-1285451374
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



