Question: Given the following equations : HBr(aq.) AHO =-120.9 kJ/mol If the Hag + Brag conventional standard enthalpy of formation of sodium ion ( AH
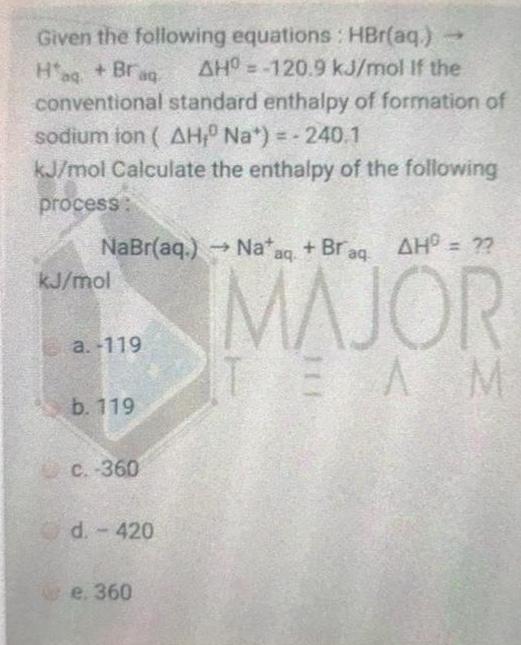
Given the following equations : HBr(aq.) AHO =-120.9 kJ/mol If the Hag + Brag conventional standard enthalpy of formation of sodium ion ( AH Na*) = - 240.1 kJ/mol Calculate the enthalpy of the following process: NaBr(aq.) Na*aq. + Br aq AH = ?? MAJOR TEAM kJ/mol a. -119 b. 119 C. -360 Od.-420 e. 360
Step by Step Solution
★★★★★
3.54 Rating (161 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (2 attachments)
63683474ee998_241791.pdf
180 KBs PDF File
63683474ee998_241791.docx
120 KBs Word File


