Answered step by step
Verified Expert Solution
Question
1 Approved Answer
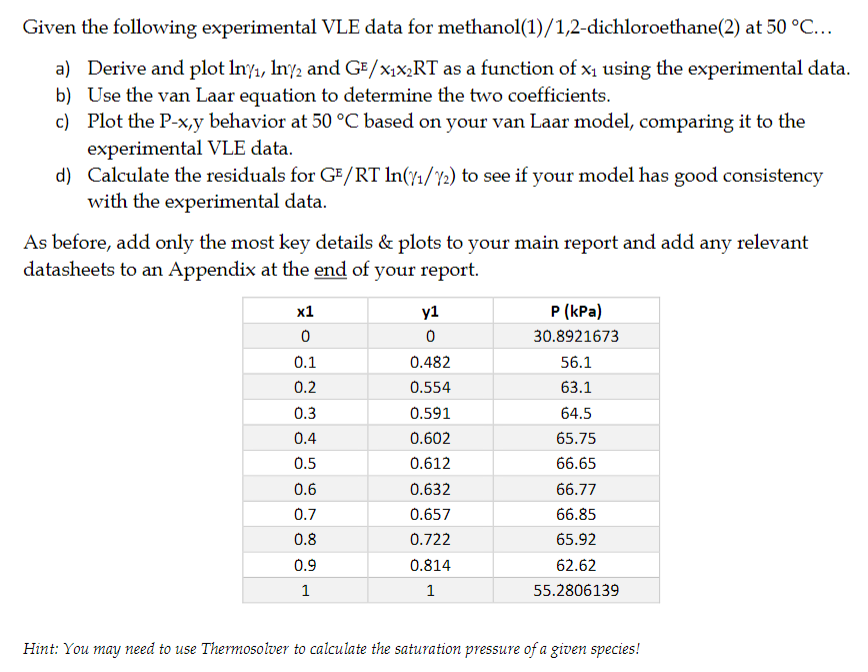
Given the following experimental VLE data for methanol ( 1 ) / 1 , 2 - dichloroethane ( 2 ) at 5 0 C .
Given the following experimental VLE data for methanoldichloroethane at
a Derive and plot and as a function of using the experimental data.
b Use the van Laar equation to determine the two coefficients.
c Plot the behavior at based on your van Laar model, comparing it to the
experimental VLE data.
d Calculate the residuals for to see if your model has good consistency
with the experimental data.
As before, add only the most key details & plots to your main report and add any relevant
datasheets to an Appendix at the end of your report.
Hint: You may need to use Thermosolver to calculate the saturation pressure of a given species!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


