Question
Given the following reactions, then arrange the metals according to their relative reactivity (from most to least)? Fe(s) + Pb2+ (aq) Fe2+(aq) + Pb(s)
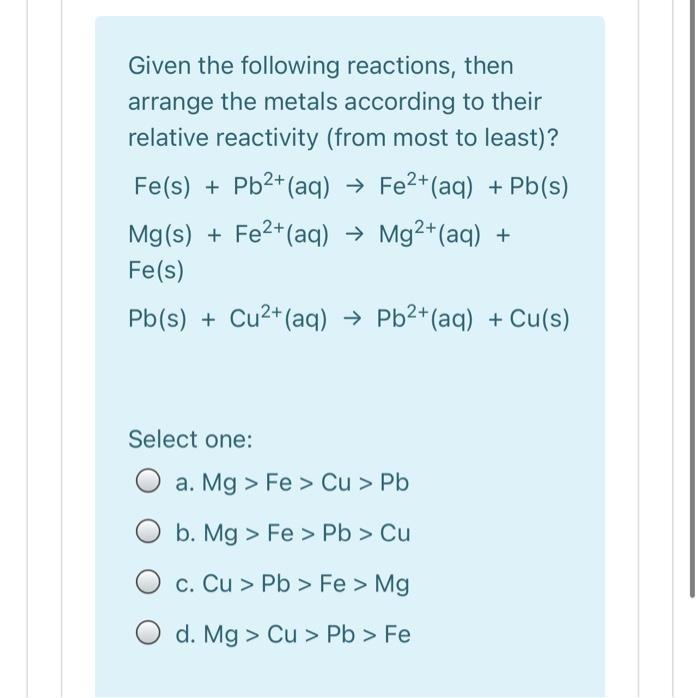
Given the following reactions, then arrange the metals according to their relative reactivity (from most to least)? Fe(s) + Pb2+ (aq) Fe2+(aq) + Pb(s) Mg(s) + Fe2+(aq) Mg2+(aq) + Fe(s) Pb(s) + Cu2+(aq) Pb2+(aq) + Cu(s) Select one: O a. Mg > Fe > Cu > Pb O b. Mg > Fe > Pb > Cu c. Cu > Pb > Fe > Mg O d. Mg > Cu > Pb > Fe
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



