Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the following thermodynamic data, calculate the solubility product of the mineral alunite KAl 3 (SO 4 ) 2 (OH) 6 at 25 o C.
Given the following thermodynamic data, calculate the solubility product of the mineral alunite KAl3(SO4)2(OH)6at 25 oC. 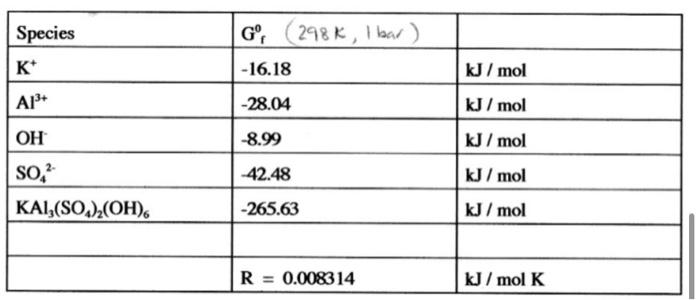
\begin{tabular}{|l|l|l|} \hline Species & Gf0(298K,1bar) & \\ \hline K+ & -16.18 & kJ/mol \\ \hline Al3+ & -28.04 & kJ/mol \\ \hline OH & -8.99 & kJ/mol \\ \hline SO42 & -42.48 & kJ/mol \\ \hline KAl3(SO4)2(OH)6 & -265.63 & kJ/mol \\ \hline & & \\ \hline & R=0.008314 & kJ/molK \\ \hline \end{tabular} 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


