Answered step by step
Verified Expert Solution
Question
1 Approved Answer
GAS LAW DIFFUSION AND GRAHAM'S LAW . INTRODUCTION LABORATORY SIMULATION 1) Given that the molar mass of ammonia is 17.030 g/mol, calculate the molar

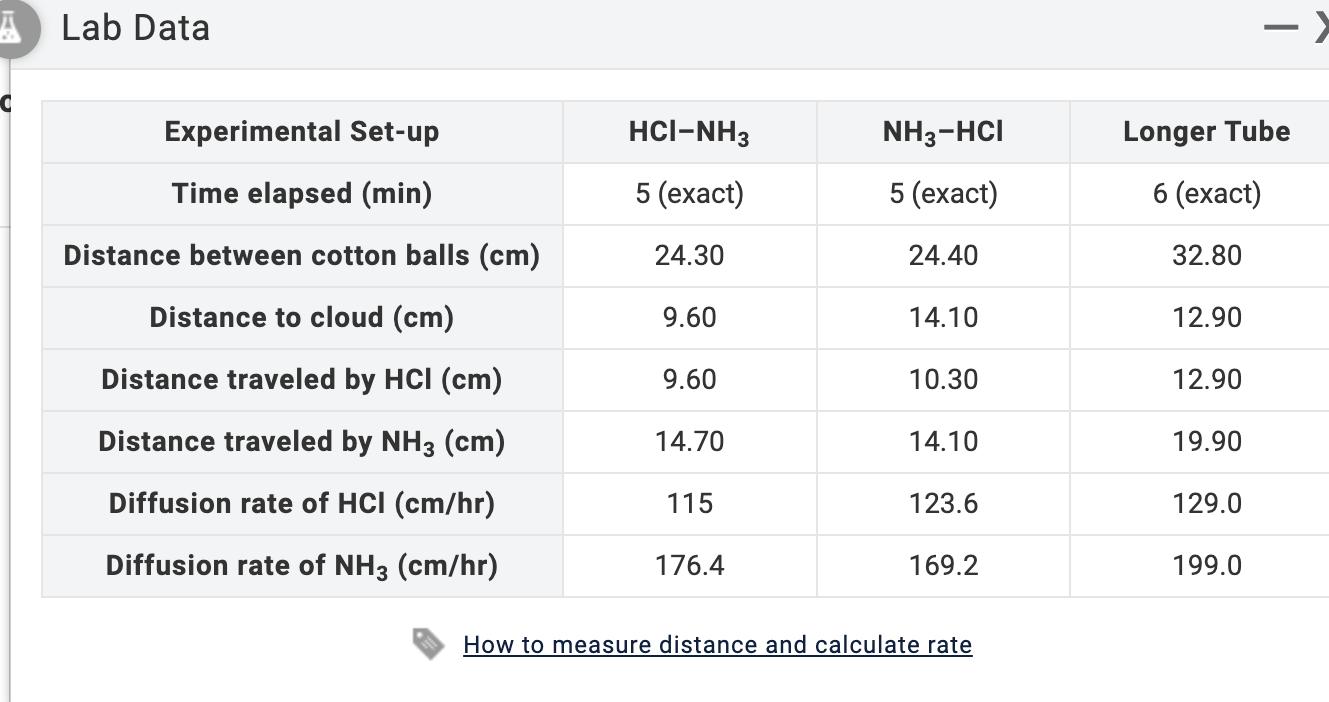
GAS LAW DIFFUSION AND GRAHAM'S LAW . INTRODUCTION LABORATORY SIMULATION 1) Given that the molar mass of ammonia is 17.030 g/mol, calculate the molar mass of hydrogen chloride using your Phase 5 (Longer Tube) Lab Data. Molar mass of HCI = g/mol ALab Data C Experimental Set-up Time elapsed (min) Distance between cotton balls (cm) Distance to cloud (cm) Distance traveled by HCI (cm) Distance traveled by NH3 (cm) Diffusion rate of HCI (cm/hr) Diffusion rate of NH3 (cm/hr) HCI-NH3 5 (exact) 24.30 9.60 9.60 14.70 115 176.4 NH3-HCI 5 (exact) 24.40 14.10 10.30 14.10 123.6 169.2 How to measure distance and calculate rate Longer Tube 6 (exact) 32.80 12.90 12.90 19.90 129.0 199.0
Step by Step Solution
★★★★★
3.33 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
By using Grahams law of diffusion the molar mass of a substance can be calculated as follows Rate 1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


