Question
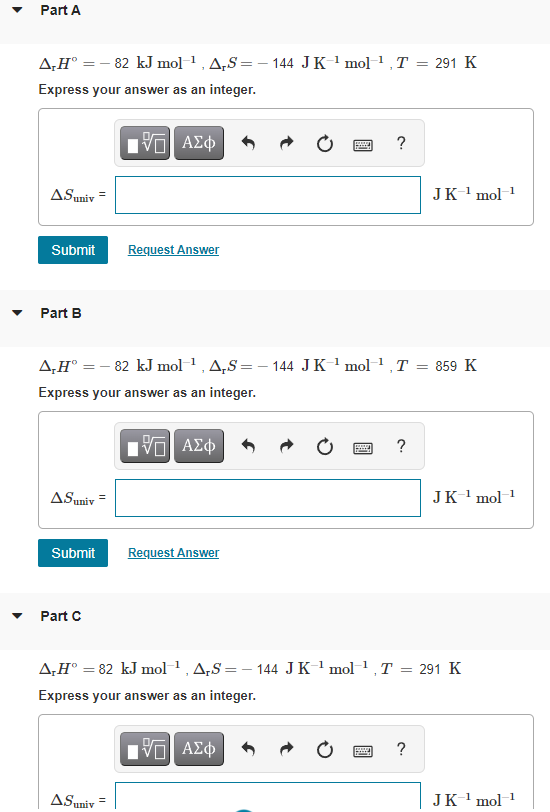
Given the values of rHrH, rSrS, and TT below, determine SunivSuniv. rH=rH= 82 kJmol1kJmol1 , rS=rS= 144 JK1mol1JK1mol1 , T=T= 291 KK Express your answer
| Given the values of rHrH, rSrS, and TT below, determine SunivSuniv. |
rH=rH= 82 kJmol1kJmol1 , rS=rS= 144 JK1mol1JK1mol1 , T=T= 291 KK Express your answer as an integer.
SubmitRequest Answer Part B rH=rH= 82 kJmol1kJmol1 , rS=rS= 144 JK1mol1JK1mol1 , T=T= 859 KK Express your answer as an integer.
SubmitRequest Answer Part C rH=rH= 82 kJmol1kJmol1 , rS=rS= 144 JK1mol1JK1mol1 , T=T= 291 KK Express your answer as an integer.
SubmitRequest Answer Part D rH=rH= 82 kJmol1kJmol1 , rS=rS= 144 JK1mol1JK1mol1 , T=T= 396 KK Express your answer as an integer.
SubmitRequest Answer Part E Predict whether or not the reaction in part A will be spontaneous.
SubmitRequest Answer Part F Predict whether or not the reaction in part B will be spontaneous.
SubmitRequest Answer Part G Predict whether or not the reaction in part C will be spontaneous.
SubmitRequest Answer Part H Predict whether or not the reaction in part D will be spontaneous.
|
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started