Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process
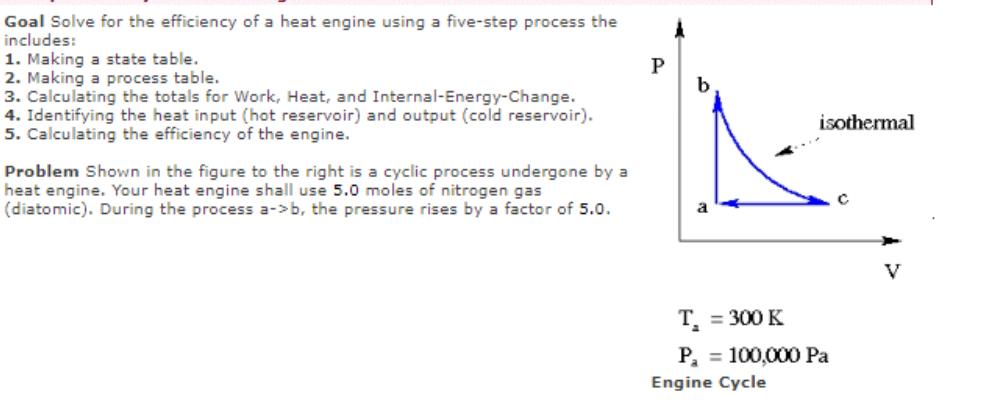
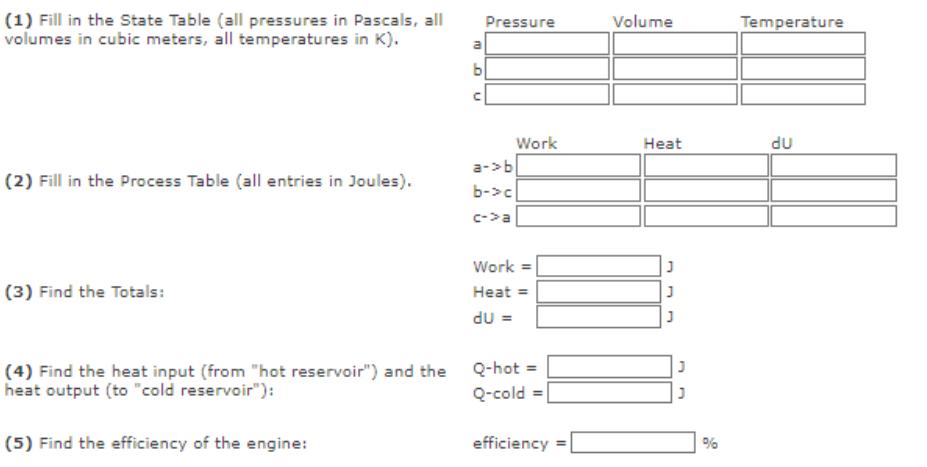
Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use 5.0 moles of nitrogen gas (diatomic). During the process a->b, the pressure rises by a factor of 5.0. P A b isothermal T = 300 K P = 100,000 Pa Engine Cycle C V (1) Fill in the State Table (all pressures in Pascals, all volumes in cubic meters, all temperatures in K). (2) Fill in the Process Table (all entries in Joules). (3) Find the Totals: a b (5) Find the efficiency of the engine: U Pressure a-> b b->c c->a Work Work = Heat = dU = (4) Find the heat input (from "hot reservoir") and the Q-hot = heat output (to "cold reservoir"): Q-cold = efficiency Volume Heat J J J % Temperature du
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Step 1 Given that The number of moles gas is n3 At state A the pressure is P A 100000 P a the temper...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


