Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Gold has always been a highly prized metal, widely used from the beginning of history as a store of value. It doesn't rust like
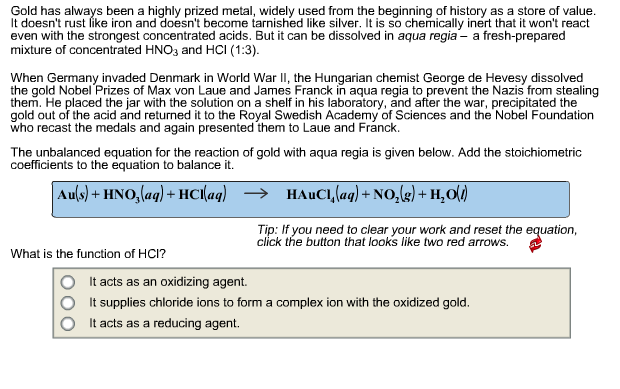
Gold has always been a highly prized metal, widely used from the beginning of history as a store of value. It doesn't rust like iron and doesn't become tarnished like silver. It is so chemically inert that it won't react even with the strongest concentrated acids. But it can be dissolved in aqua regia- a fresh-prepared mixture of concentrated HNO3 and HCI (1:3). When Germany invaded Denmark in World War II, the Hungarian chemist George de Hevesy dissolved the gold Nobel Prizes of Max von Laue and James Franck in aqua regia to prevent the Nazis from stealing them. He placed the jar with the solution on a shelf in his laboratory, and after the war, precipitated the gold out of the acid and returned it to the Royal Swedish Academy of Sciences and the Nobel Foundation who recast the medals and again presented them to Laue and Franck. The unbalanced equation for the reaction of gold with aqua regia is given below. Add the stoichiometric coefficients to the equation to balance it. Au(s) + HNO3(aq) + HCl(aq) What is the function of HCI? HAUCI, (aq) + NO(g) + HO(l) Tip: If you need to clear your work and reset the equation, click the button that looks like two red arrows. It acts as an oxidizing agent. It supplies chloride ions to form a complex ion with the oxidized gold. It acts as a reducing agent.
Step by Step Solution
★★★★★
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Solution Function of HCl ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


