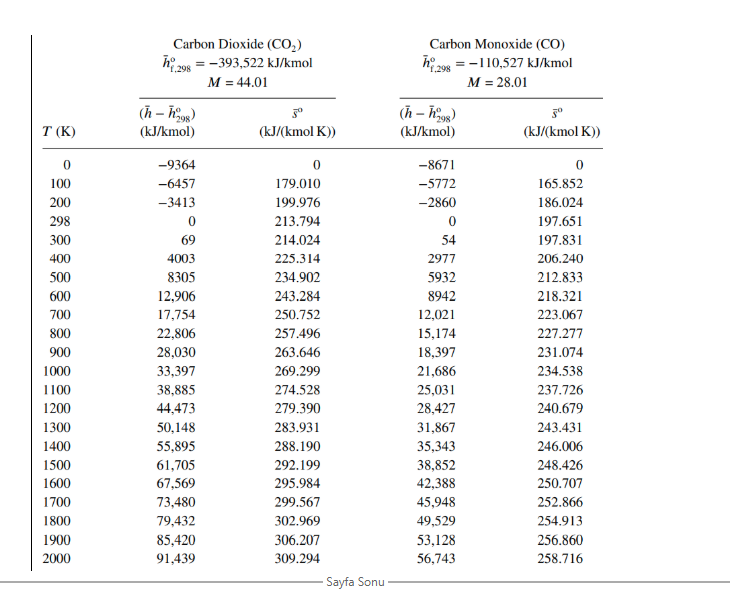
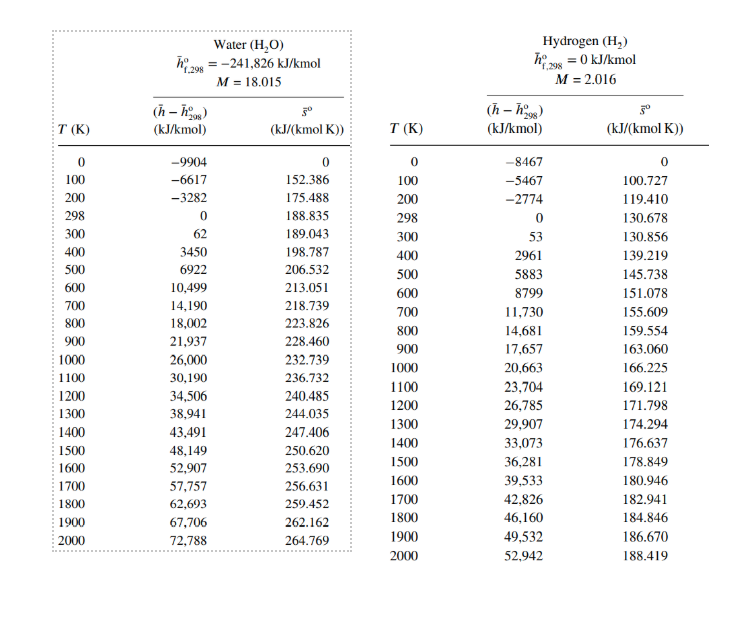
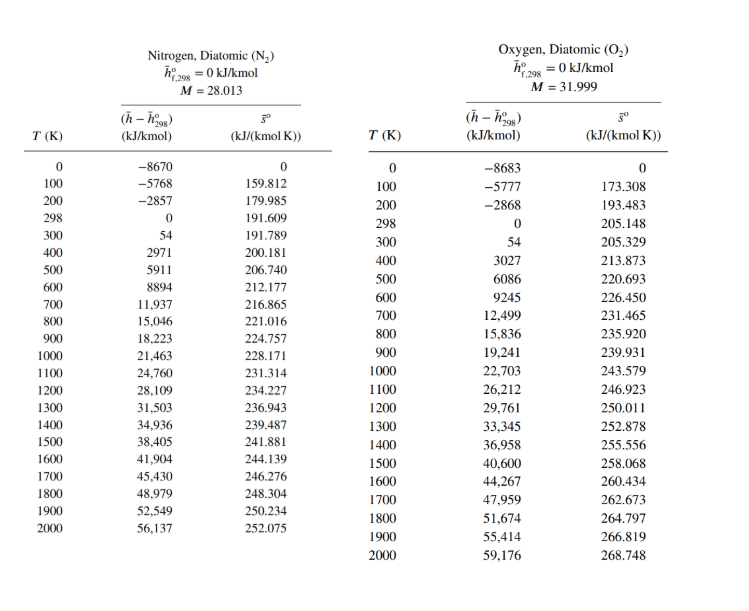
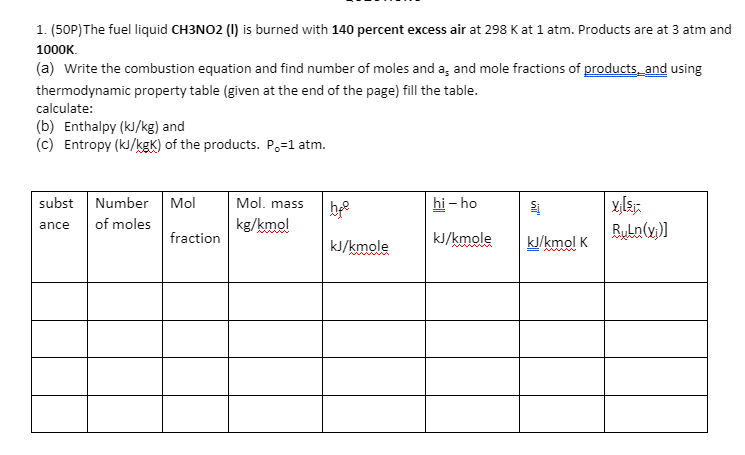
h24 = = Carbon Dioxide (CO2) :-393,522 kJ/kmol M = 44.01 (n-ngs) 30 (kJ/kmol) (kJ/(kmol K)) Carbon Monoxide (CO) hay = -110,527 kJ/kmol f.298 M = 28.01 (h-h98) 3 (kJ/kmol) (kJ/(kmol K)) T(K) 0 100 200 298 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 -9364 -6457 -3413 0 69 4003 8305 12,906 17,754 22,806 28,030 33,397 38,885 44.473 50,148 55,895 61,705 67,569 73,480 79,432 85,420 91,439 0 179.010 199.976 213.794 214.024 225.314 234.902 243.284 250.752 257.496 263.646 269.299 274.528 279.390 283.931 288.190 292.199 295.984 299.567 302.969 306.207 309.294 Sayfa Sonu -8671 -5772 -2860 0 54 2977 5932 8942 12,021 15,174 18,397 21,686 25,031 28,427 31,867 35,343 38,852 42,388 45,948 49,529 53,128 56,743 0 165.852 186.024 197.651 197.831 206.240 212.833 218.321 223.067 227.277 231.074 234.538 237.726 240.679 243.431 246.006 248.426 250.707 252.866 254.913 256.860 258.716 Water (H,0) 298 = -241,826 kJ/kmol M = 18.015 Hydrogen (H) 198 = 0 kJ/kmol M = 2.016 f.298 (n-08) T(K) 5 (kJ/(kmol K)) (h - h) (kJ/kmol) (kJ/kmol) T (K) (kJ/(kmol K)) 0 100 200 298 300 400 500 600 0 100 200 298 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 -9904 -6617 -3282 0 62 3450 6922 10,499 14,190 18,002 21,937 26,000 30,190 34,506 38,941 43,491 48,149 52,907 57,757 62,693 67,706 72,788 0 152.386 175.488 188.835 189.043 198.787 206.532 213.051 218.739 223.826 228.460 232.739 236.732 240.485 244.035 247.406 250.620 253.690 256.631 259.452 262.162 264.769 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 -8467 -5467 -2774 0 53 2961 5883 8799 11,730 14,681 17,657 20,663 23,704 26,785 29,907 33,073 36,281 39,533 42,826 46,160 49,532 52,942 0 100.727 119.410 130.678 130.856 139.219 145.738 151.078 155.609 159.554 163.060 166.225 169.121 171.798 174.294 176.637 178.849 180.946 182.941 184.846 186.670 188.419 Oxygen, Diatomic (02) h 298 = 0 kJ/kmol M = 31.999 Nitrogen, Diatomic (N) h = 0 kJ/kmol M = 28.013 (h-1998) 3 (kJ/kmol) (kJ/(kmol K)) (n-09) T(K) T(K) (kJ/kmol) 30 (kJ/(kmol K)) 0 100 200 298 300 400 0 100 200 298 300 400 500 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 -8670 -5768 -2857 0 54 2971 5911 8894 11,937 15,046 18,223 21,463 24,760 28,109 31.503 34,936 38,405 41,904 45.430 48.979 52,549 56,137 0 159.812 179.985 191.609 191.789 200.181 206.740 212.177 216.865 221.016 224.757 228.171 231.314 234.227 236.943 239.487 241.881 244.139 246.276 248.304 250.234 252.075 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 -8683 -5777 -2868 0 54 3027 6086 9245 12,499 15,836 19,241 22,703 26,212 29,761 33,345 36,958 40,600 44,267 47,959 51,674 55,414 59,176 0 173.308 193.483 205.148 205.329 213.873 220.693 226.450 231.465 235.920 239.931 243.579 246.923 250.011 252.878 255.556 258.068 260.434 262.673 264.797 266.819 268.748 1800 1900 2000 1. (50P)The fuel liquid CH3NO2 (1) is burned with 140 percent excess air at 298 Kat 1 atm. Products are at 3 atm and 1000K (a) Write the combustion equation and find number of moles and a, and mole fractions of products and using thermodynamic property table (given at the end of the page) fill the table. calculate: (b) Enthalpy (kJ/kg) and (C) Entropy (kJ/kgk) of the products. Po=1 atm. bre hi - ho subst Number Mol ance of moles fraction Mol, mass kg/kmo! yilsio ByLn(x;)] kJ/kmole kJ/kmole kJ/kmol K










