Answered step by step
Verified Expert Solution
Question
1 Approved Answer
have been struggling with these 3 questions, answers with explanation would be appreciated a The work function () for a metal is 7.60x10-19 J. What


have been struggling with these 3 questions, answers with explanation would be appreciated
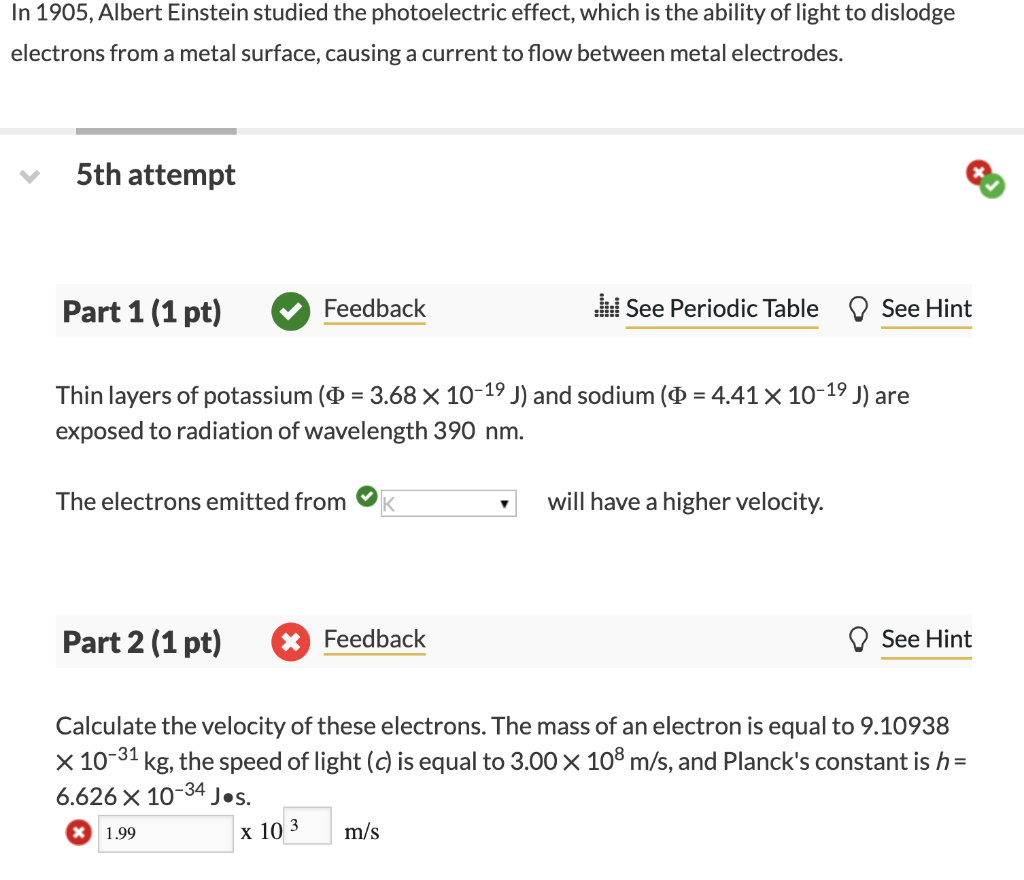
a The work function () for a metal is 7.60x10-19 J. What is the longest wavelength of electromagnetic radiation that can eject an electron from the surface of a piece of the metal? nm In 1905, Albert Einstein studied the photoelectric effect, which is the ability of light to dislodge electrons from a metal surface, causing a current to flow between metal electrodes. 5th attempt Part 1 (1 pt) Feedback W See Periodic Table See Hint = Thin layers of potassium (0 = 3.68 X 10-19 J) and sodium (Q = 4.41 x 10-19 J) are exposed to radiation of wavelength 390 nm. The electrons emitted from will have a higher velocity. Part 2 (1 pt) X Feedback See Hint Calculate the velocity of these electrons. The mass of an electron is equal to 9.10938 x 10-31 kg, the speed of light (c) is equal to 3.00 x 108 m/s, and Planck's constant is h= 6.626 X 10 -34 Jos. m/s X 1.99 X 10 3 Write all the possible sets of magnetic quantum numbers, me for an electron in the n =3 shell that have an angular momentum quantum number b=1 and a spin quantum number ms = +1/2. Express your response in the form, for example, -1,0,+1 with no spaces between numbers, signs indicated, and negative numbers before zero or positive numbers. -1,0,1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


