Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In Season 1 of Breaking Bad, Walter White confronts Tuco Salamanca with fulminated mercury. Fulminated mercury Hg(CNO), is primarily used as a trigger for
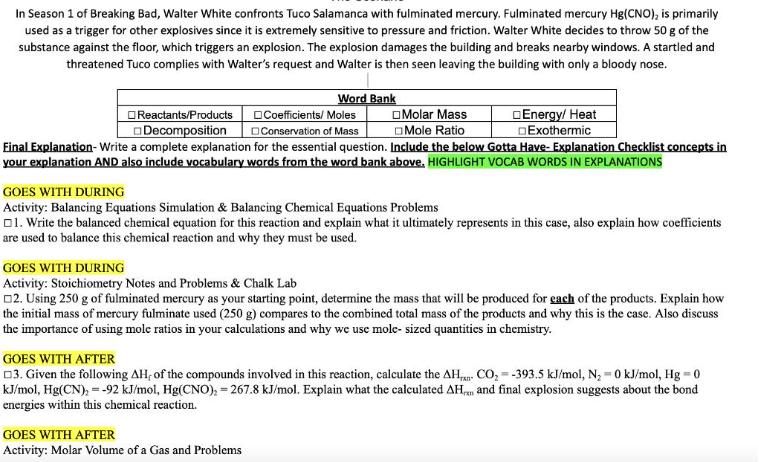
In Season 1 of Breaking Bad, Walter White confronts Tuco Salamanca with fulminated mercury. Fulminated mercury Hg(CNO), is primarily used as a trigger for other explosives since it is extremely sensitive to pressure and friction. Walter White decides to throw 50 g of the substance against the floor, which triggers an explosion. The explosion damages the building and breaks nearby windows. A startled and threatened Tuco complies with Walter's request and Walter is then seen leaving the building with only a bloody nose. Reactants/Products Decomposition Word Bank Coefficients/Moles Conservation of Mass Final Explanation- Write a complete explanation for the essential question. Include the below Gotta Have- Explanation Checklist concepts in your explanation AND also include vocabulary words from the word bank above. HIGHLIGHT VOCAB WORDS IN EXPLANATIONS Molar Mass Mole Ratio Energy/ Heat Exothermic GOES WITH DURING Activity: Balancing Equations Simulation & Balancing Chemical Equations Problems 1. Write the balanced chemical equation for this reaction and explain what it ultimately represents in this case, also explain how coefficients are used to balance this chemical reaction and why they must be used. GOES WITH AFTER Activity: Molar Volume of a Gas and Problems GOES WITH DURING Activity: Stoichiometry Notes and Problems & Chalk Lab 2. Using 250 g of fulminated mercury as your starting point, determine the mass that will be produced for each of the products. Explain how the initial mass of mercury fulminate used (250 g) compares to the combined total mass of the products and why this is the case. Also discuss the importance of using mole ratios in your calculations and why we use mole- sized quantities in chemistry. GOES WITH AFTER 3. Given the following AH, of the compounds involved in this reaction, calculate the AH kJ/mol, Hg(CN), = -92 kJ/mol, Hg(CNO), 267.8 kJ/mol. Explain what the calculated AH energies within this chemical reaction. CO-393.5 kJ/mol, N - 0 kJ/mol, Hg = 0 and final explosion suggests about the bond
Step by Step Solution
★★★★★
3.48 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
Answer 1 2HgCNO2 2CO2 N2 Hg HgCN2 The decomposition of mercuryII fulminate yields carbon dioxide gas ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


