Answered step by step
Verified Expert Solution
Question
1 Approved Answer
heat capacity of liquid benzene is 1 3 3 and Tm = 1 5 0 0 , a = - 3 3 . 8 9
heat capacity of liquid benzene is and Tm a b c d
Please do the following before coming to class on Monday to help us solve the problem using different methods:
Heat capacity of liquid benzene is Jg K
Calculate hh in KJMol
Calculate heat of vaporization of benzene at T
Calculate van Der waals constants a and bTc and Pc can be obtained from relevant table sent to you.
Calculate vapor volume at state and
Integrate Cpvapor dT and determine the value.
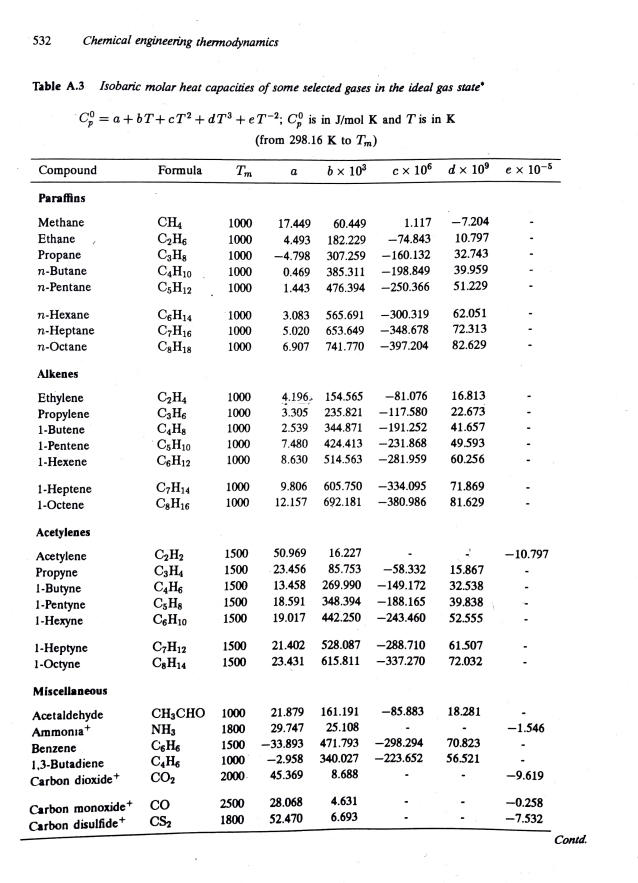
The Cp of benzene vapor can be obtained from table below.
EManePropanenButanenPentanenHexanenHeptanenOctaneAlkenesEthylenePropyleneButenePenteneHexeneHepteneOcteneAcetylenesAcetylenePropyneButynePentyneHexyneHeptyneOctyneMiscellaneousAcetaldehydeAmmonatBenzeneCsHCHCsHCHCHCHsChHCsHeC,HCsHCHiCHCgHChHaCjH,CAHsC&HsCeHhoCHCHNHCeHsCHCHCHO y S
Chemical engineering thermodynamics
Table A Isobaric molar heat capacities of some selected gases in the ideal gas state
; is in olK and is in from to
tableCompoundFormula,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


