Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(Heisenberg) A carbon-14 nucleus decays into nitrogen-14, emitting in the process an electron and a neutrino (this process is relevant for carbon dating, as
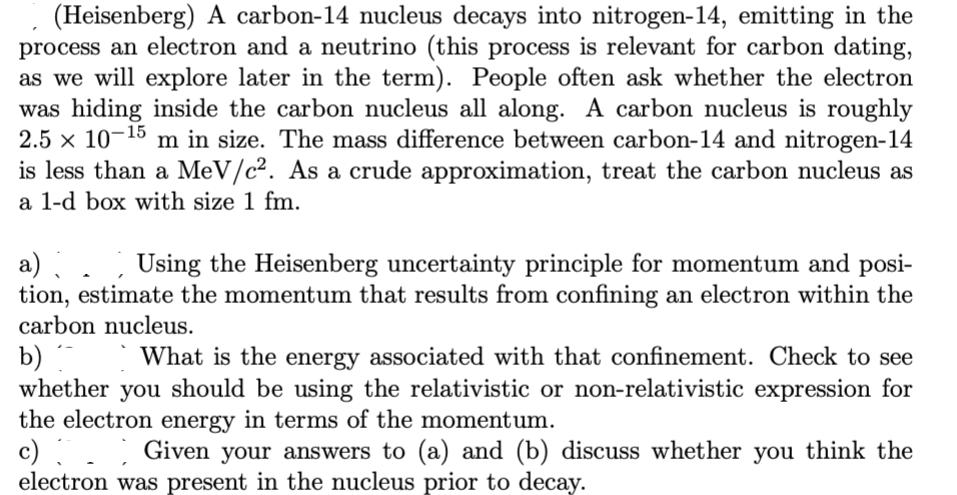
(Heisenberg) A carbon-14 nucleus decays into nitrogen-14, emitting in the process an electron and a neutrino (this process is relevant for carbon dating, as we will explore later in the term). People often ask whether the electron was hiding inside the carbon nucleus all along. A carbon nucleus is roughly 2.5 x 10-15 m in size. The mass difference between carbon-14 and nitrogen-14 is less than a MeV/c. As a crude approximation, treat the carbon nucleus as a 1-d box with size 1 fm. a) Using the Heisenberg uncertainty principle for momentum and posi- tion, estimate the momentum that results from confining an electron within the carbon nucleus. b) What is the energy associated with that confinement. Check to see whether you should be using the relativistic or non-relativistic expression for the electron energy in terms of the momentum. Given your answers to (a) and (b) discuss whether you think the electron was present in the nucleus prior to decay.
Step by Step Solution
★★★★★
3.54 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
a Using the Heisenberg Uncertainty Principle the uncertainty in position x and momentum p of the ele...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


