Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hello. Can you write out the answers for both questions and explain how you got the answer be using the key words in your sentence


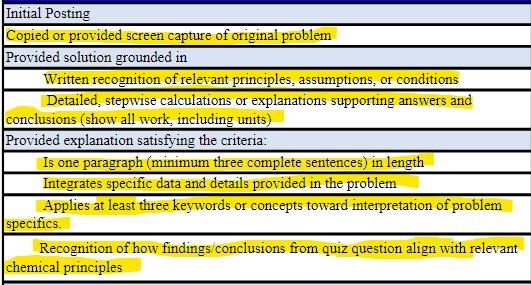
Hello. Can you write out the answers for both questions and explain how you got the answer be using the key words in your sentence and also follow the rubric that I have highlighted for each of the answer please!
Assuming all have the same principal-quantum number (n) value and are found in a multi-electron atom, which is lowest in energy? Keywords: wavefunction, orbital, subshell, potential energy, principal quantum number, angularmomentum quantum number, degenerate/degeneracy, probability Concepts: Quantum numbers, orbitals, potential energy Group of answer choices A B C D Which has a maximum of three mi values? Keywords: angular-momentum quantum number, magnetic quantum number, orientation, orbital, subshell, degenerate, probability Concepts: quantum numbers Group of answer choices A B Question 32.54 pts What is the de Broglie wavelength of a C60 molecule (m=1.201024kg) traveling at 220m/s?(1m=1x 1012pm)? Keywords: mass, deBroglie wavelength, velocity, momentum, wavelength, Joule, Planck constant Concepts: wave-particle duality, factors influencing wave properties of matter Group of answer choices 2.52pm 2.52m 1.20pm 5.22m Initial Posting Copied or provided screen capture of original problem Provided solution grounded in Written recognition of relevant principles, assumptions, or conditions Detailed, stepwise calculations or explanations supporting answers and conclusions (show all work, including units) Provided explanation satisfying the criteria: Is one paragraph (minimum three complete sentences) in length Integrates specific data and details provided in the problem Applies at least three keywords or concepts toward interpretation of problem specifics. Recognition of how findings/conclusions from quiz question align with relevant chemical principlesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


