Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hello! could you please let me know if my equation is right for #8 and then solve #9, 10 and 11?? thank you. 8. The
hello! could you please let me know if my equation is right for #8 and then solve #9, 10 and 11?? thank you.

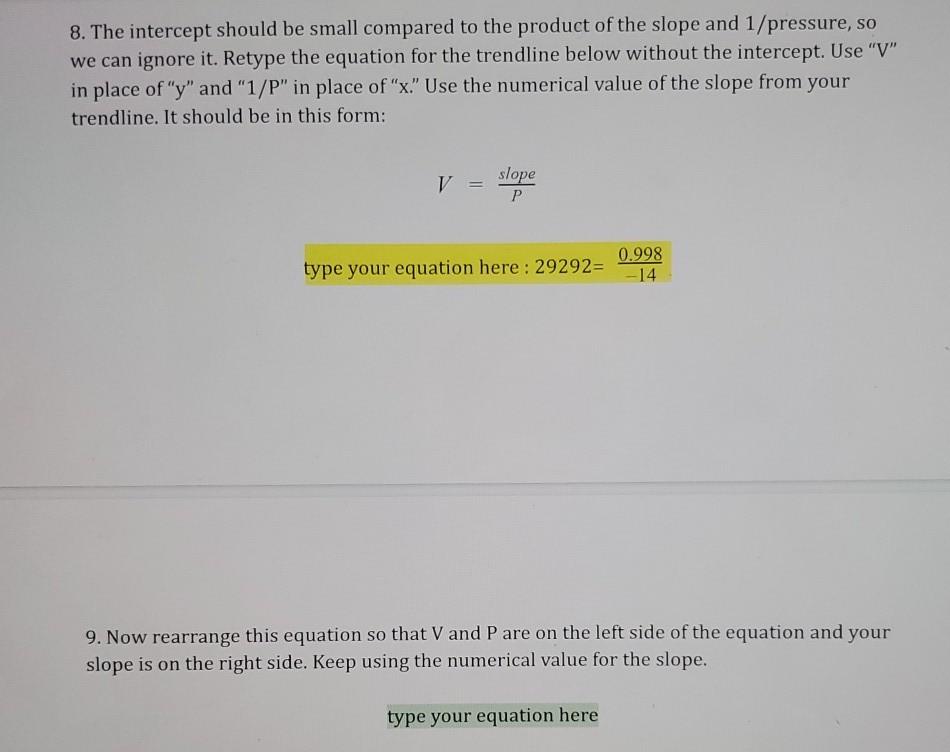
8. The intercept should be small compared to the product of the slope and 1/pressure, so we can ignore it. Retype the equation for the trendline below without the intercept. Use "V" in place of "y" and "1/P" in place of "x." Use the numerical value of the slope from your trendline. It should be in this form: V = slope P 0.998 type your equation here: 29292= -14 9. Now rearrange this equation so that V and P are on the left side of the equation and your slope is on the right side. Keep using the numerical value for the slope. type your equation here 9. Now rearrange this equation so that V and P are on the left side of the equation and your slope is on the right side. Keep using the numerical value for the slope. type your equation here 10. Suppose there is a system with a certain initial volume (V1) and initial pressure (P.). Keeping the moles of gas particles and temperature constant, it is then changed to a final volume (V2) and final pressure (P.). Write the equation above twice: once for the initial conditions and once for the final conditions: type your first equation here type your second equation here 11. Finally, to get a simple gas law relating volume and pressure, we note that the right sides of the two equations above are identical. If the right sides are equal, that means the left sides are also equal. Write an equation showing the left side of the first equation being equal to the left side of the second equation: type your simple gas law here This should be the same as the simple gas law relating pressure and volume you saw in lecture
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


