Question
In the training, a supervisor has asked you to do regular checking of combustion process. The checking is done by analyzing meter reading from instrument
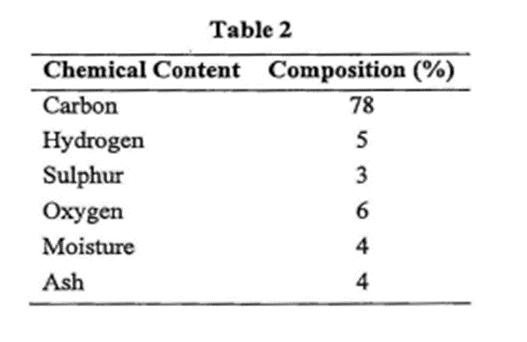
In the training, a supervisor has asked you to do regular checking of combustion process. The checking is done by analyzing meter reading from instrument panel. The instrument panel for combustion product indicated that 02 meter is 12% and C02 meter is 18%. The C02 meter also absorbs S02. Therefore, the C02 meter reading includes percentage of S02. Coal is used as fuel for boiler combustion and the ultimate analysis of the coal is shown in Table 2.
The combustion of air has dry bulb temperature of 27 °C and wet bulb temperature of 21 °C. The flue (exhaust) gas temperature is 250 °C and boiler room temperature is 30 °C. By assume air absolute humidity to be 0.0132 kg moisture per kg air and Cp for.gas exhaust to be 1.10 kJ/kg K, you need to provide a report on analysis of the following parameters to the supervisor.
a) The complete chemical equation for the combustion process.
b) The percentage of air excess.
c) The energy carried by dry flue gas per kg fuel.
d) The energy loss due to moisture in the air supply per kg fuel.
Table 2 Chemical Content Composition (%) Carbon Hydrogen Sulphur Oxygen Moisture Ash 78 5 3 6 4 4
Step by Step Solution
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


