Hello, I wanted to check my work here I am having doubt if I did it correct or not thanks!

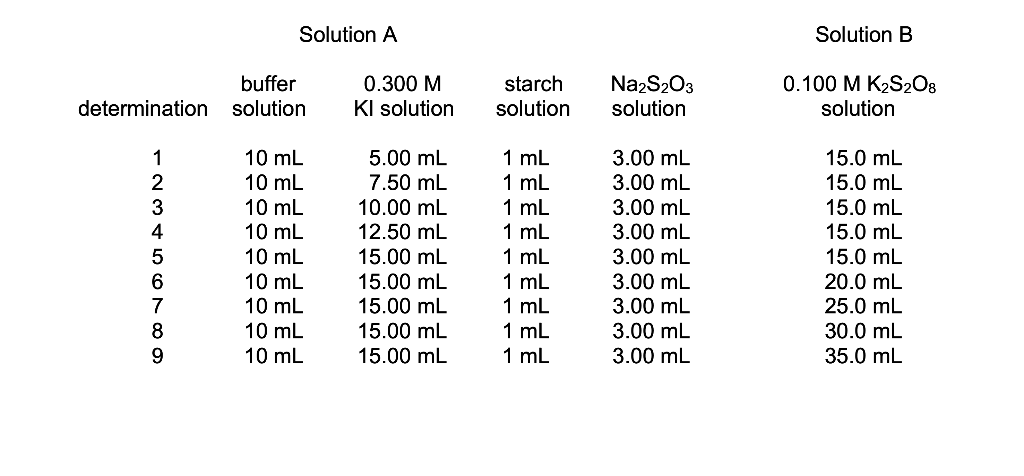
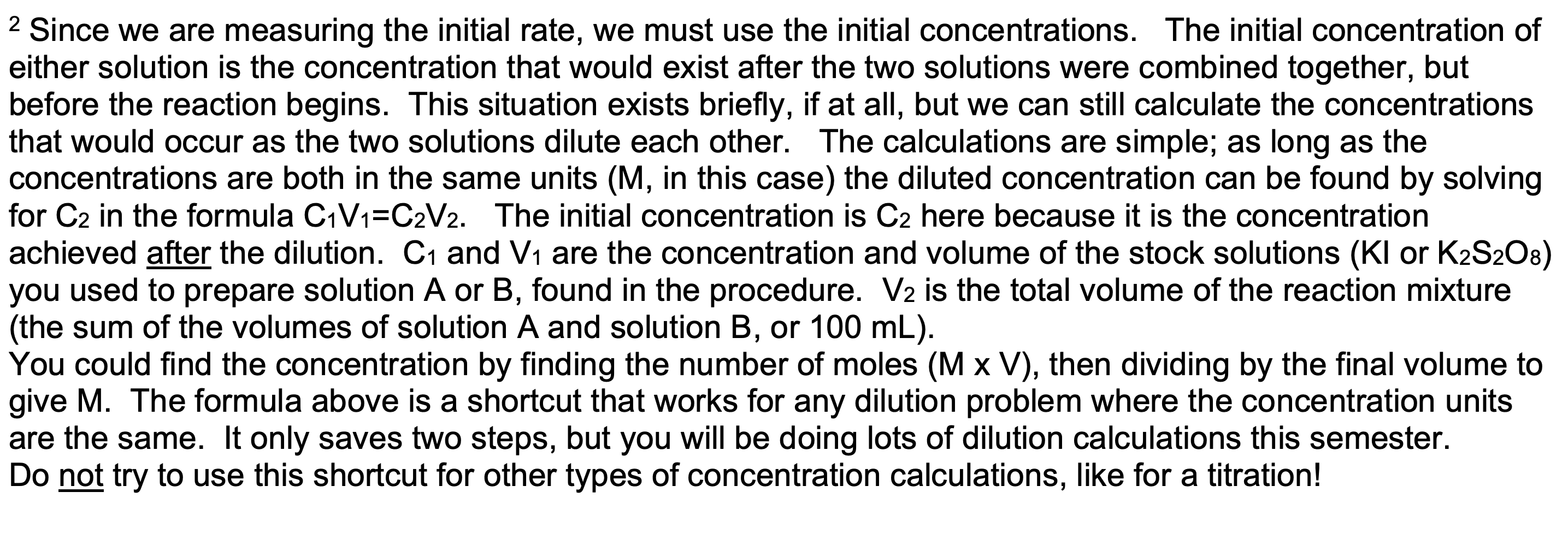
5. Fill in the values below and calculate [1-]i, the initial concentration of - for Determination 1. (This is the concentration after the reagents have mixed but before the reaction begins, referred to here as C2.) See Table 1 in the procedure to find the volume of 0.300 M KI used. The total final volume of the solution is 100.0 mL. See footnote 2 on p. 6 for help. (2 pt.) C1 = V1 = C2 = V2 = Solution A Solution B buffer determination solution 0.300 M Kl solution starch solution Na2S2O3 solution 0.100 MK2S2O8 solution 1 2 3 4 5 6 7 8 9 10 mL 10 mL 10 mL 10 mL 10 mL 10 mL 10 mL 10 mL 10 ml 5.00 mL 7.50 mL 10.00 mL 12.50 mL 15.00 mL 15.00 mL 15.00 mL 15.00 mL 15.00 mL 1 mL 1 mL 1 mL 1 mL 1 mL 1 mL 1 mL 1 mL 1 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 3.00 mL 15.0 mL 15.0 mL 15.0 mL 15.0 mL 15.0 mL 20.0 mL 25.0 mL 30.0 mL 35.0 mL 3 3 2 Since we are measuring the initial rate, we must use the initial concentrations. The initial concentration of either solution is the concentration that would exist after the two solutions were combined together, but before the reaction begins. This situation exists briefly, if at all, but we can still calculate the concentrations that would occur as the two solutions dilute each other. The calculations are simple; as long as the concentrations are both in the same units (M, in this case) the diluted concentration can be found by solving for C2 in the formula C1V1=C2V2. The initial concentration is C2 here because it is the concentration achieved after the dilution. C1 and V1 are the concentration and volume of the stock solutions (Kl or K2S208) you used to prepare solution A or B, found in the procedure. V2 is the total volume of the reaction mixture (the sum of the volumes of solution A and solution B, or 100 mL). You could find the concentration by finding the number of moles (M x V), then dividing by the final volume to give M. The formula above is a shortcut that works for any dilution problem where the concentration units are the same. It only saves two steps, but you will be doing lots of dilution calculations this semester. Do not try to use this shortcut for other types of concentration calculations, like for a titration









