Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hello please work out homework for me , please number each question and write clear as possible using BEARD MARIANNA J Numbers. use Beard Marianna
hello please work out homework for me , please number each question and write clear as possible using BEARD MARIANNA J Numbers. 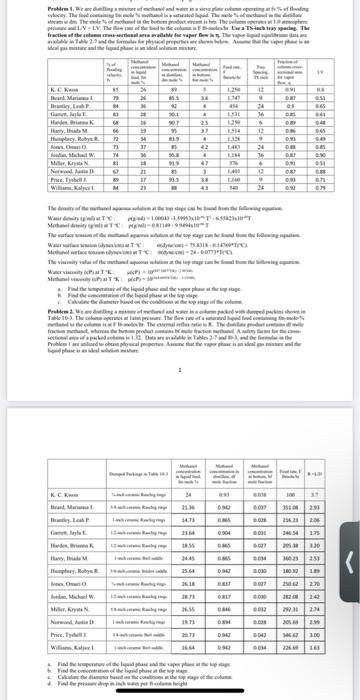

use Beard Marianna J numbers to complete! 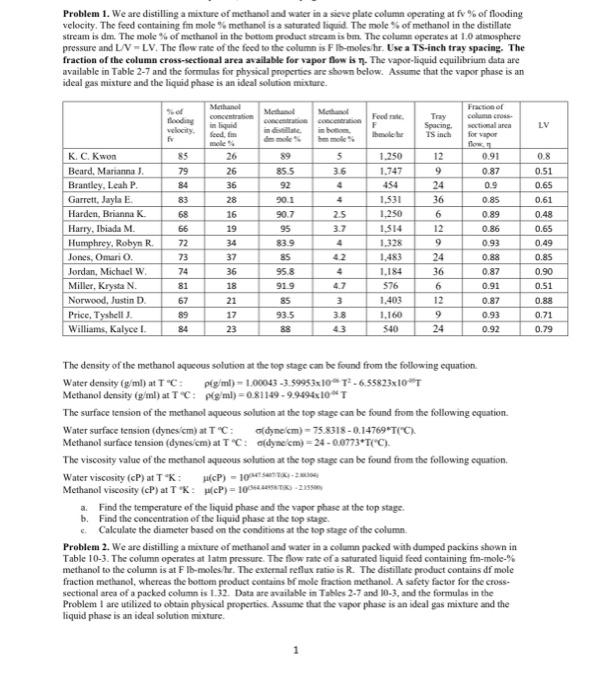
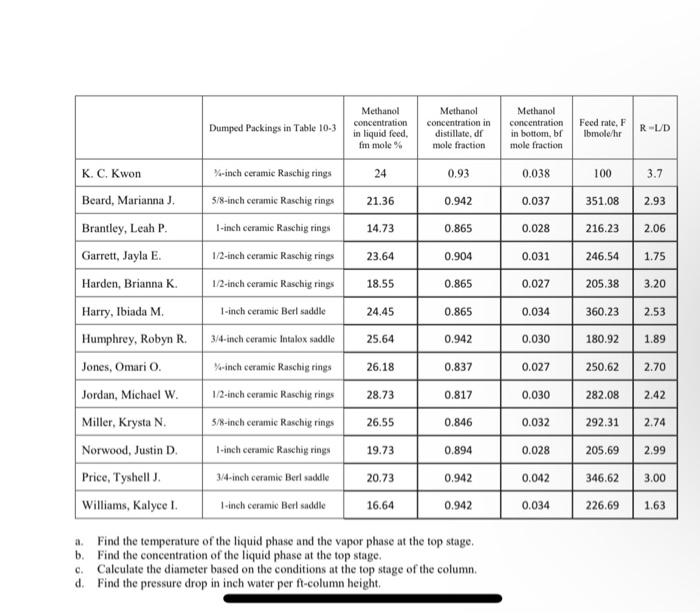
htial akar . The tead as as LIV - LY, TMs the real state. La Tate Iratist dails late Real pr aparil p |KE hari Mama t D 28 N M 15 114 DET 03 24 - Thala Ila. It LE - - - ON DOS 23 7 N ! LI N , MEN Nr. has. The W OM 27 NI - 21 11 21 1 4 47 3 33 . 00 - an O. Tamle WartiyaTTI 10. - 19 TM ) . 34. Tie viarasati wary Marthaal ) - Prathikalai paiduatipala T Th a malee | 34 K.CA lanari Mania 21 3.00 5.00 | tels name llars, Iniah M| 3029 23.2020 317 23.30 23 233 30 23 2012 45 EMA . DAI 2 | E BN 30 yaN | 65 Maa haaaa his, iyar williams, PN 1.00 22 - Tales in allpa - Problem 1. We are distilling a mixture of methanol and water in a sieve plate column operating at fv % of flooding velocity. The feed containing fm mole % methanol is a saturated liquid. The mole of methanol in the distillate stream is dm. The mole % of methanol in the bottom product stream is bm. The column operates at 1.0 atmosphere pressure and L/V-LV. The flow rate of the feed to the column is Fib-moleshr. Use a TS-inch tray spacing. The fraction of the column cross-sectional area available for vapor flow is T. The vapor-liquid equilibrium data are available in Table 2-7 and the formulas for physical properties are shown below. Assume that the vapor phase is an ideal gas mixture and the liquid phase is an ideal solution mixture. Methanol Metal Fraction of concentration Food Try flooding column in liquid F Spacing social area inte feed Ihme TS inch for LV f 85 79 84 5 3.6 4 18 4 K. C. Kwon Beard, Marianna). Brantley, Leah P. Garrett, Jayla E Harden, Brianna K Harry, Ibiada M. Humphrey, Robyn R Jones, Omari O. Jordan, Michael W Miller, Krysta N. Norwood, Justin D Price, Tyshell. Williams, Kalyce ! WIM 26 26 36 28 16 19 34 37 36 18 21 89 855 92 901 90.7 95 83.9 85 95.8 919 85 93.5 88 83 68 66 72 73 74 81 67 89 84 25 3.7 4 42 w 1.250 1.747 454 1.531 1.250 1.514 1.328 1.483 1.184 576 1.403 1.160 540 12 9 24 36 6 12 9 24 36 6 12 9 24 0.91 0.87 09 0.85 0.89 0.86 0.93 0.88 0.87 0.91 0.87 0.93 0.92 0.8 0.51 0.65 0.61 0.48 0.65 0.49 0.85 0.90 0.51 0.88 0.71 0.79 4 SI 47 17 3.8 43 23 The density of the methanol aqueous solution at the top stage can be found from the following equation Water density (g/ml) at T"C: plg/ml) - 1.00043 -3.59953x10T-6.55823x10"T Methanol density (g/ml) at TC: Pig ml) = 0.81149 - 9.9494x10T The surface tension of the methanol aqueous solution at the top stage can be found from the following equation Water surface tension (dynes cm) at TC: odyne cm) - 75.8318 -0.14769*T("C). Methanol surface tension (dynes cm) at T'C: odyme cm) = 24 -0.0773T(C). The viscosity value of the methanol aqueous solution at the top stage can be found from the following cquation. Water viscosity (CP) at TK: #cP) - 10 Methanol viscosity (CP) at TK: (P) = 100STK-21 a Find the temperature of the liquid phase and the vapor phase at the top stage. b. Find the concentration of the liquid phase at the top stage e Calculate the diameter based on the conditions at the top stage of the column. Problem 2. We are distilling a mixture of methanol and water in a column packed with dumped packins shown in Table 10-3. The column operates at lat pressure. The flow rate of a saturated liquid feed containing fm-mole-% methanol to the column is at Fil-moleshur. The external reflux ratio is R. The distillate product contains df mole fraction methanol, whereas the bottom product contains bf mole fraction methanol. A safety factor for the cross- sectional area of a packed column is 1.32. Data are available in Tables 2.7 and 10-3, and the formulas in the Problem I are utilized to obtain physical properties. Assume that the vaper phase is an ideal gas mixture and the liquid phase is an ideal solution mixture. 1 Dumped Packings in Table 10-3 Methanol concentration in liquid feed, fm mole % Methanol concentration in distillate, dr mole fraction Methanol concentration in bottom, br mole fraction Feed rate, F Ibmole/hr R-UD 24 0.93 0.038 100 3.7 %-inch ceramic Raschig rings 5/8-inch ceramic Raschig rings 1-inch ceramic Raschig ring 21.36 0.942 0.037 2.93 14.73 0.865 0.028 2.06 1/2-inch ceramic Raschig rings 23.64 0.904 0.031 1.75 18.55 0.865 0.027 3.20 K. C, Kwon Beard, Marianna J. Brantley, Leah P. Garrett, Jayla E Harden, Brianna K. Harry, Ibiada M Humphrey, Robyn R. Jones, Omario. Jordan, Michael W. Miller, Krysta N. Norwood, Justin D. 24.45 0.865 0.034 2.53 25.64 0.942 0.030 1.89 351.08 216.23 246.54 205,38 360.23 180.92 250.62 282.08 292.31 205.69 346.62 226.69 26.18 0.837 1/2-inch ceramic Raschig rings 1-inch ceramic Berl saddle 3/4-inch ceramic Intalox saddle Yo-inch ceramic Raschig rings 1/2-inch ceramic Raschig rings 5/8-inch ceramic Raschig rings 1-inch ceramic Raschig rings 3/4-inch ceramic Berl saddle 1-inch ceramic Berl saddle 2.70 28.73 0.817 2.42 0.027 0.030 0.032 0.028 26.55 0.846 2.74 19.73 0.894 2.99 20.73 0.942 0.042 3.00 Price, Tyshell J. Williams, Kalyce I 16.64 0.942 0.034 1.63 a Find the temperature of the liquid phase and the vapor phase at the top stage. b. Find the concentration of the liquid phase at the top stage. c. Calculate the diameter based on the conditions at the top stage of the column. d. Find the pressure drop in inch water per ft-column height 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


