Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help (1) The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight
help (1) 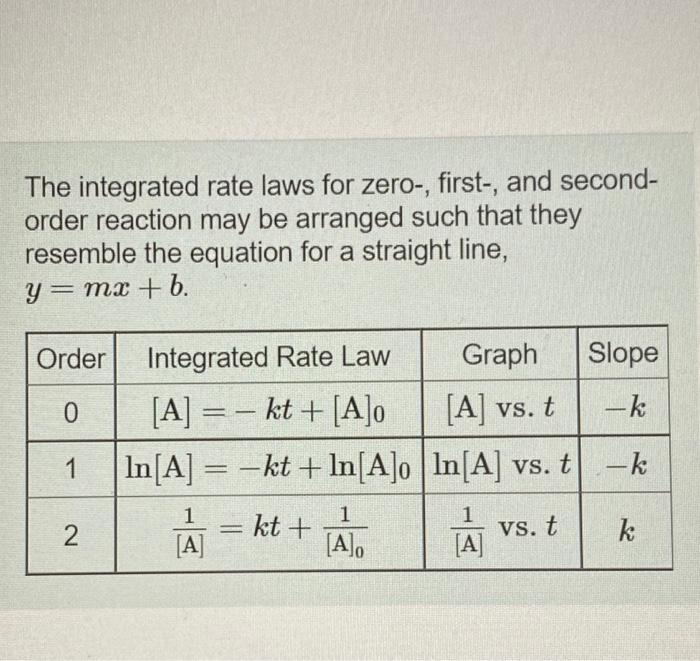
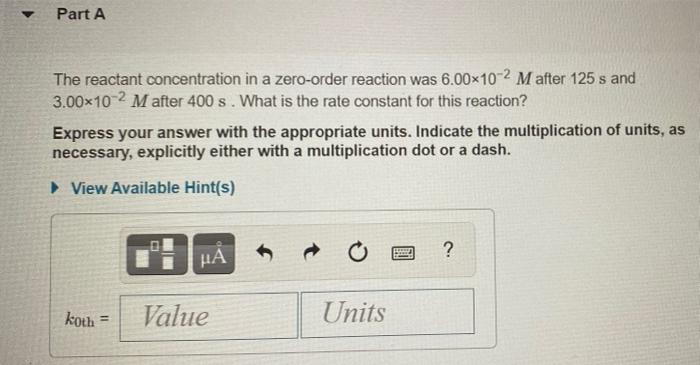
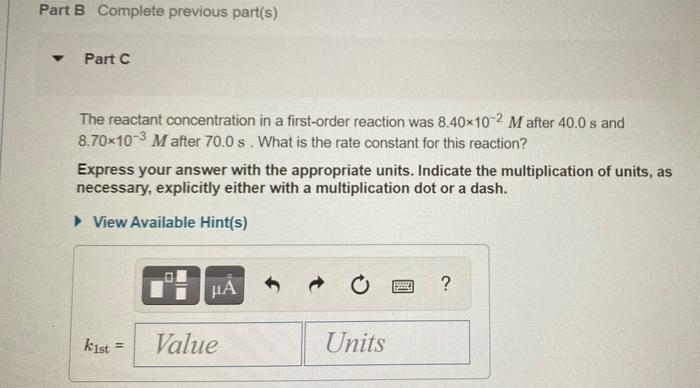
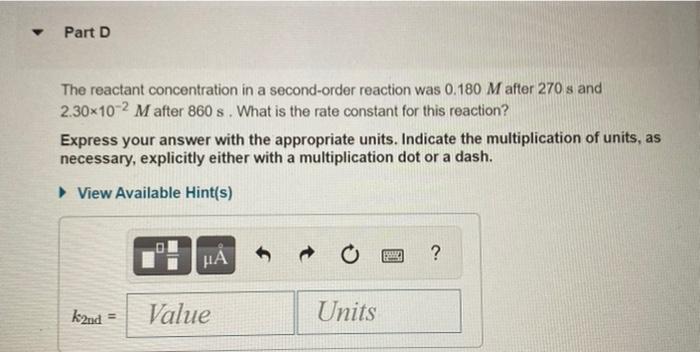
The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight line, y = mx + 6. Order Integrated Rate Law Graph Slope 0 [A] =- kt + [A]o [A] vs. t -k 1 In[A] = -kt + In[A]o In[A] vs. t -k 1 - 1 1 [A] = kt + 1 [A] vs. t k [A], N Part A The reactant concentration in a zero-order reaction was 6.00x10-2 M after 125 s and 3.00x10-2 M after 400 s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. View Available Hint(s) ? koth = Value Units Part B Complete previous part(s) Part C The reactant concentration in a first-order reaction was 8.40x10-2 M after 40.0 s and 8.70x10-3 M after 70.0 s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. a View Available Hint(s) BRE ? kist Value Units Part D The reactant concentration in a second-order reaction was 0.180 M after 270 s and 2.30-10-2 M after 860 s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. View Available Hint(s) o HA ? k2nd = Value Units 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


