Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help explain everything asap Consider the following mechanism: ES1 CIO(aq) + H2O(l) = = HClO(aq) + OH(aq) (fast) ES2 (aq) + HClO(aq) -HIO(aq) + Cl(aq)
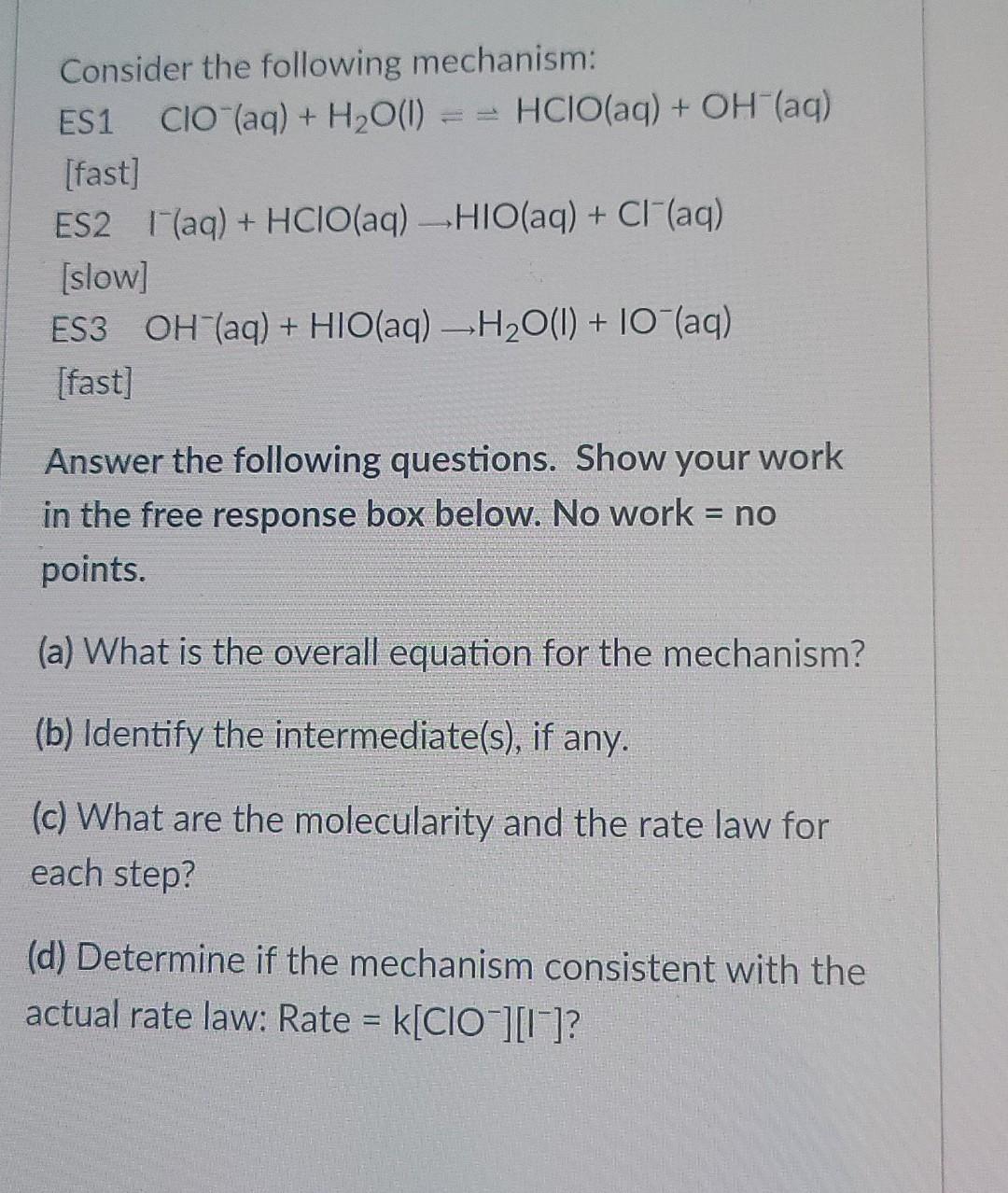
help explain everything asap
Consider the following mechanism: ES1 CIO"(aq) + H2O(l) = = HClO(aq) + OH(aq) (fast) ES2 (aq) + HClO(aq) -HIO(aq) + Cl(aq) [slow] ES3 OH(aq) + HIO(aq) -H2O(l) + 10 (aq) [fast] Answer the following questions. Show your work in the free response box below. No work = no points. = (a) What is the overall equation for the mechanism? (b) Identify the intermediate(s), if any. (c) What are the molecularity and the rate law for each step? (d) Determine if the mechanism consistent with the actual rate law: Rate = k[ClO][15]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


