I need help on this asap

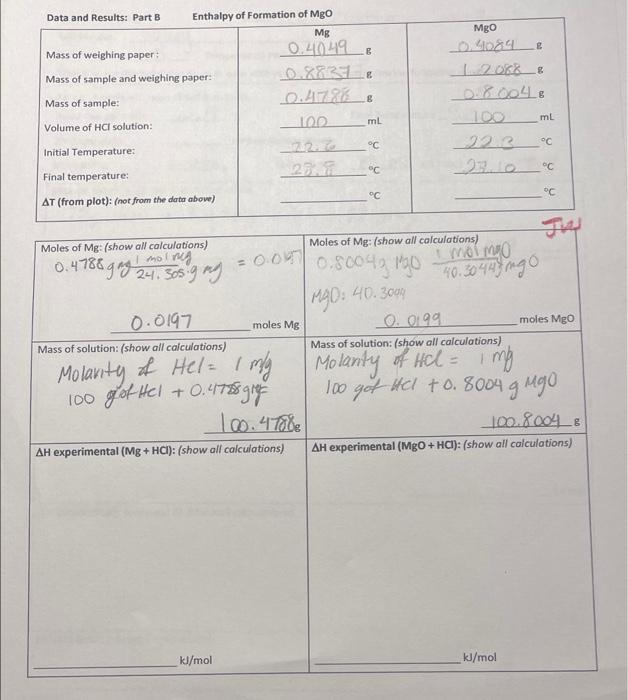
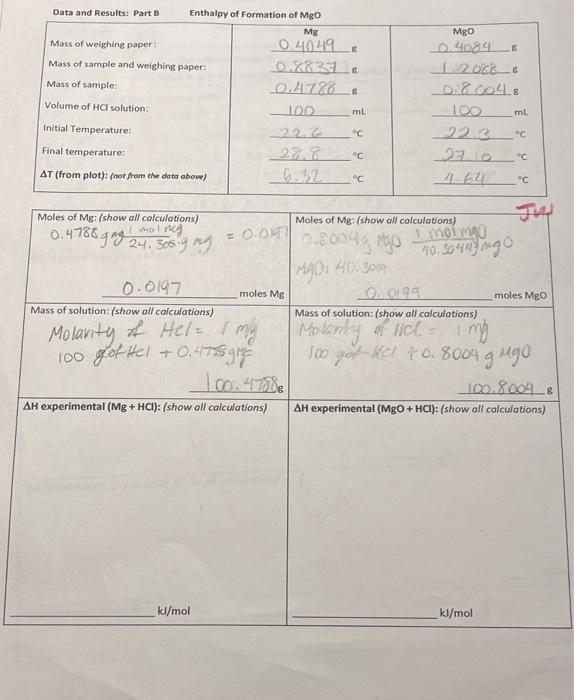
E Data and Results: Part 8 Enthalpy of Formation of Mgo Mg Mass of weighing paper 0.40.49 & Mass of sample and weighing paper: Mass of sample: Volume of HCl solution: 1002 ml C 22 Initial Temperature: MgO _09084 12068__& 0.8.004 6 100 22.3 ml C c C Final temperature: C c C AT (from plot): (not from the data above) Moles of Mg: (show all calculations) 1 mol ng 0.4788 g mg 24. 305.9 mg 0.00 Moles of Mg: (show all calculations) Momo 0.8004grgo 40.30448 ingo 1990: 40.3000 moles Mg moles Mgo 0.0197 Mass of solution: (show all calculations) 0.0199 Mass of solution: (show all calculations) = Molarity of Hel= 1 mly Molanity of Hel = 1 mg 1 100 got Hel +0.4788 gig 100 got HCl +0.8004 g Mgo 10.4T08 100.80048 AH experimental (Mg + HCI): (show all calculations) AH experimental (MgO + HCI): (show all calculations) kl/mol kJ/mol Part A Reaction 1: Heat of Solution KNO, Initial Temperature _225_ Final Temperature_135_" AT (from plot) not from the data above) H2O volume: 27 L (+0.5 mL) 2010 Mass KNO: TW Mass of solution (g): (show all calculations) 75g H2O + 7.0 140g & NO2 = 82.0142 8.014 AH experimental: (show all calculations) kJ/mol AH (theoretical): (see page 8) (show all calculations) kJ/mol Percent Error: (show all calculations) Chem. 1E Thermochemistry Experiment 6 Hess's Law Calculation: Refer to reactions in introduction AH" Me(s) Halal ".. MO(s) HCl(aq) AH", -285 9 kl/mol Hle). XO, H200 (heat of formation of water) Combine AH.. AH, AH' using Hess's law to find AH (MgO): Mels) 0:1) MO(s) SHOW YOUR WORK HERE: Calculated AHMgo kJ/mol Il/mol Theoretical value for AH, MgO (Text or CRC handbook): Percent Error Experimental vs. Theoretical: Lab Partner: Name: Lab: Section: Score: /50 (Staple this page to the top of your data sheets and plots) Turn in this page with pages 9, 10, 11, and 12 along with your plots of temperature vs. time by the next lab meeting. Each student must produce their own graphs. The determination of AT from each plot must be clearly labeled on each plot. Each plot must be titled labeled appropriately. Points will be deducted if this is not so! Summary and Results: Part A Reaction 1: Heat of Solution KNO, AH (Experimental) AH (theoretical) % error Part A Reaction 2: Heat of Solution Neutralization AH (Experimental) AH (theoretical) % error Part B: Enthalpy of formation of magnesium Oxide AH (Experimental) AH (theoretical) % error Instructor Comments: Chem. 1E Thermochemistry Data and Results: Part A Reaction 2: Heat of Neutralization M (HCI): M(NaOH) Volume HCl(aq): (0.5 mL) Volume NaOH(aq): (+0.5 mL) Initial Temperature: 22.0_c Final Temperature: AT (from plot) fnot from the date above) TLD Mass of Solution: (show all calculations) molarity of HCL = of NOOH = 1 509 HCl +44 2. Nacht Moles :show all calculations) Moles OH (show all calculations) m: volume -> moles-malu Volume 50 x1 = 50 moles H 495 moles OH AH experimental: (show all calculations) Molarity in moles 435 NON kl/mol AH (theoretical): (see page 8) (show all calculations) kl/mol Percent Error: (show all calculations) Hess's Law Calculation: Refer to reactions in introduction. Mg(s) + HCl(aq) *: ".. MgO(s) + HCl(aq) AH', -285.9 kl/mol Hz(e)%0.(c) H2O(n) (heat of formation of water) Combine AH", AH', & AH', using Hess's law to find A" (MBO): Mg(s) + XO.(e) MgO(s) SHOW YOUR WORK HERE: Calculated AHMgO: kJ/mol kJ/mol Theoretical value for AH: MgO (Text or CRC handbook): Percent Error Experimental vs. Theoretical: Experiment 6 Data and Results: Part A Reaction 2: Heat of Neutralization M (HCI): M(NaOH) Volume HCl(a): (10.5 mL) Volume NaOH(aq) Initial Temperature: 22.6_ Final Temperature: AT (from plot) 6.5 not from the date showe) Mass of Solution: (show all calculations) 1 NAOH 49.5 10.5 mL) 29.4 Jus molanty of HC mella linty 50 g Hel +49 59 NOH= 99.59. 99.5 Molarity Moles H":show all calculations) males m. volume -> Moles : morto volume 50x1= 50 50 moles H AH experimental: (show all calculations) Moles OH show all calculations) Vies 1495 WON moles OH kl/mol AH (theoretical): (see page 8) (show all calculations) kl/mol Percent Error: (show all calculations) E Data and Results: Part 3 Enthalpy of Formation of MgO Mg Mass of weighing paper 0.4049 Mass of sample and weighing paper: 0.86331 Mass of sample: 0.4726_8 Volume of HCl solution: 100 mt Initial Temperature: _22.6 Final temperature: 200 Mgo 0.4084 2018_ 0. 2004, 100 ml 22.3 AT (from plot): (not from the data abow) C 4.62 C Moles of Mg: (show all calculations) molres Moles of Mg: (show all calculations) 0.4786 s gag 24.30s gay = 0.01 7.80043 Mo no mgo 40.30445 ngo M40. 40.30m 0.0197 moles Mg moles Mgo LO006 Mass of solution: (show all calculations) Mass of solution: (show all calculations) 1 Molarity of Hel= my Moranty of Ilcl- ling lich 100 got Hel + 0.4175 g 100 got ko +0.8004 g ugo. 100.8004& AH experimental (MgO + HCI): (show all calculations) AH experimental (Mg + HCI): (show all calculations) kJ/mol kl/mol Turn this and the following pages in only with your plots. This lab is due the follow Part A Reaction 1: Heat of Solution KNO, Initial Temperature 22.54 Final Temperature_18-5_ AT (from plot) -4.12_not from the date above) Mass KNOS: 7.0.140 H2O volume: 15_ML (+0.5 ml) TV Mass of solution (s): (show all calculations) 75g H2O +7.0140g KNO3 = 82.0142 AH experimental: (show all calculations) kl/mol AH (theoretical): (see page 8) (show all calculations) kJ/mol Percent Error: (show all calculations)