Answered step by step
Verified Expert Solution
Question
1 Approved Answer
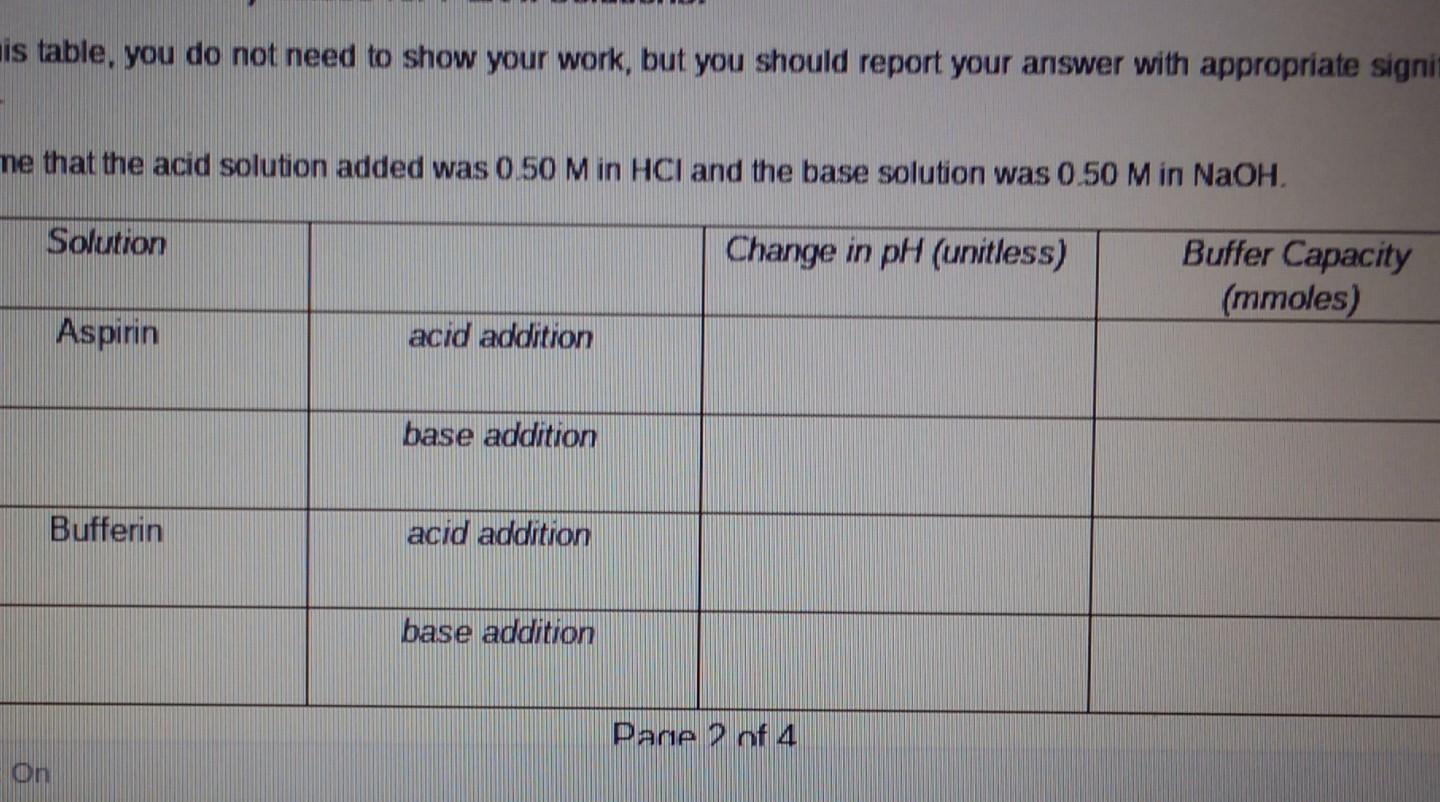
help is table, you do not need to show your work, but you should report your answer with appropriate signi me that the acid solution
help

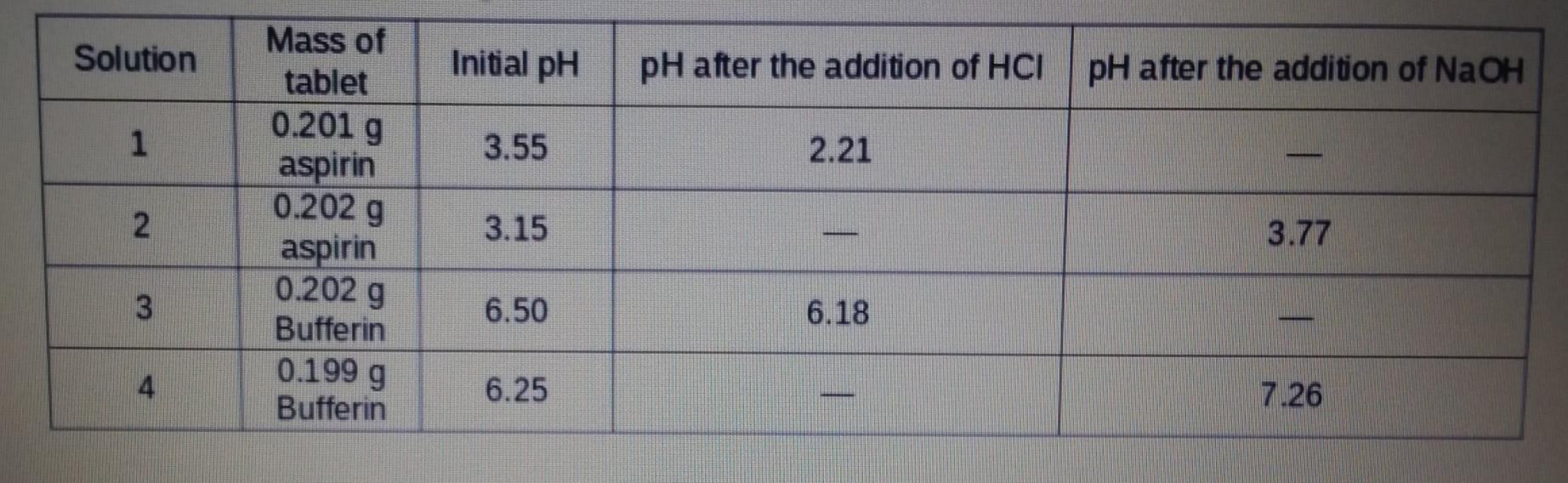
is table, you do not need to show your work, but you should report your answer with appropriate signi me that the acid solution added was 0 50 Min HCl and the base solution was 0.50 M in NaOH. Solution Change in pH (unitless) Buffer Capacity (mmoles) Aspirin acid addition base addition Bufferin acid addition base addition Pane Pof 4 On Solution Initial pH pH after the addition of HCI pH after the addition of NaOH 1 3.55 2.21 2 Mass of tablet 0.201 g aspirin 0.202 g aspirin 0.202 g Bufferin 0.1999 Bufferin 3.15 3.77 3 6.50 6.18 4 6.25 . 7.26 Photos showing labels of generic aspirin (left) and a generic form of Bufferin (right) tablet packaging Aspirin 325 Tri-Buffered Aspirin 325
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


