Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help me!!! 5. The Redich-Kiwong equation of state is given by where R-universal gas constant [ = 0.518 kJ/(kg-K), T = absolute temperature (K), p
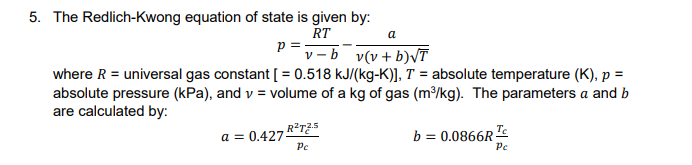
Help me!!!
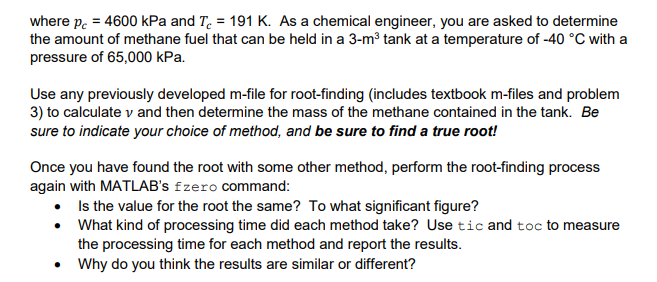
5. The Redich-Kiwong equation of state is given by where R-universal gas constant [ = 0.518 kJ/(kg-K), T = absolute temperature (K), p absolute pressure (kPa), and vvolume of a kg of gas (m/kg). The parameters a and b are calculated by: b = 0.0866 Pc Pc where pe4600 kPa and T191 K. As a chemical engineer, you are asked to determine the amount of methane fuel that can be held in a 3-m3 tank at a temperature of -40 C with a pressure of 65,000 kPa. Use any previously developed m-file for root-finding (includes textbook m-files and problem 3) to calculate v and then determine the mass of the methane contained in the tank. Be sure to indicate your choice of method, and be sure to find a true root! Once you have found the root with some other method, perform the root-finding process again with MATLAB's fzero command . Is the value for the root the same? To what significant figure? . What kind of processing time did each method take? Use tic and toc to measure the processing time for each method and report the results . Why do you think the results are similar or differentStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


