Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me answer this in 15 minutes in a hurry to submit im struggling please help 32. Sulfuric acid dissolves aluminum metal according to the
help me answer this in 15 minutes in a hurry to submit im struggling please help 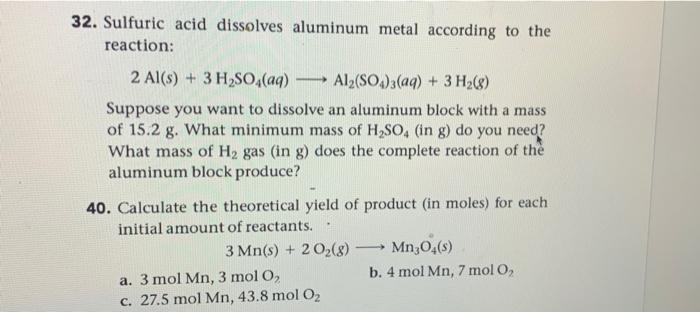
32. Sulfuric acid dissolves aluminum metal according to the reaction: 2Al(s)+3H2SO4(aq)Al2(SO4)3(aq)+3H2(g) Suppose you want to dissolve an aluminum block with a mass of 15.2g. What minimum mass of H2SO4 (in g) do you need? What mass of H2 gas (in g ) does the complete reaction of the aluminum block produce? 40. Calculate the theoretical yield of product (in moles) for each initial a mount of reactants. 3Mn(s)+2O2(g)Mn3O4(s) a. 3molMn,3molO2 b. 4molMn,7molO2 c. 27.5molMn,43.8molO2 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


