Question
Complete the table. Temperature Trial 1 Trial 2 Trial 3 Concentration of iodide solution (mM) 100.0 100.0 100.0 Concentration of thiosulfate solution (mM) 20.1 20.1
Complete the table.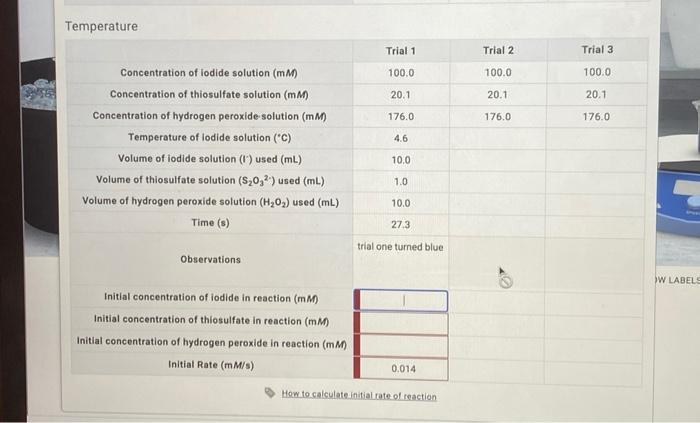
Temperature Trial 1 Trial 2 Trial 3 Concentration of iodide solution (mM) 100.0 100.0 100.0 Concentration of thiosulfate solution (mM) 20.1 20.1 20.1 Concentration of hydrogen peroxide solution (mM) 176.0 176.0 176.0 Temperature of lodide solution ("C) 4.6 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (S20,2) used (mL) 1.0 Volume of hydrogen peroxide solution (H202) used (mL) 10.0 Time (s) 27.3 trial one turned blue Observations W LABELS Initial concentration of iodide in reaction (mM) Initial concentration of thiosulfate in reaction (mM) Initial concentration of hydrogen peroxide in reaction (mM) Initial Rate (mM/s) 0.014 How to calculate.initial rate of.reaction
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Linear Algebra A Modern Introduction
Authors: David Poole
4th edition
1285463242, 978-1285982830, 1285982835, 978-1285463247
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



