Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help me i'm confuse 4. What is the value of the siope for the relationship between mass of chlorate and volume of oxygen generated? (1pts)

Help me i'm confuse
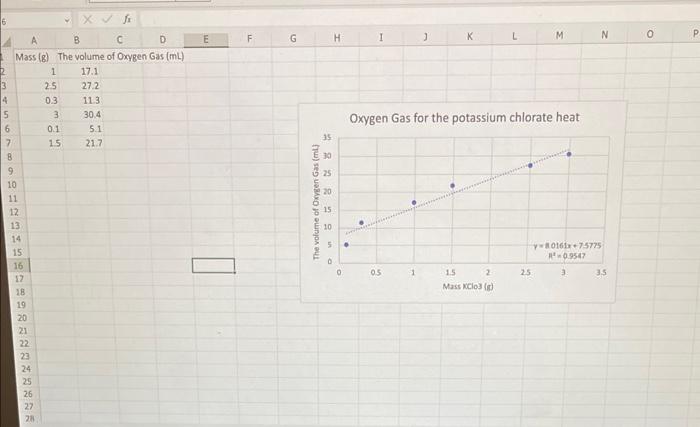
4. What is the value of the siope for the relationship between mass of chlorate and volume of oxygen generated? (1pts) Slope (mu/g of KClO3) 5. The eqation for determining percent error is % error =ActualActual-Experimental100 (1pts) Use 8.3mL/g for the "Actual value and determine the percent error: 4. Error 6. Use your computer-generated graph to answer the following three questions. (1pts) a. Visually estimate the volume, in milliliters, of oxygen gas produced when 2.70g of potassium chlorate is heated under the same conditions. Volume of oxygen gas (mL) (1pts) b. Calculate the volume of gas using the equation of the best fit line. Use the graph to ensure that this value is reasonable. Volume of oxygen gas (mL) c. Compare the calculated gas volume to the two visually interpolated values (Steps 2 and 6a ). Briefly discuss any discrepancies. 7. Using your computer-generated graph: (1pts) Visually estimate the mass, in grams, of KClO3 needed to generate 20.0mL of oxygen gas. a. Mass of KClO3(g) (1pts) b. Calculate the mass, in grams, of potassium chlorate needed to generate 20.0mL of oxygen gas. Use the graph to ensure that this value is reasonable. Mass of KClO3(g) (1pts) Compare the calculated mass of potassium chlorate to the two visually interpolated values (Steps 2 and 7a ). Briefly discuss any discrepancies Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


