Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me please! H-C-OH 3. Let's now consider a slightly different reaction, a reaction in solution: Glucose (aq) Fructose (aq) CHOH Glucose Fructose 0.0 Ho
help me please! 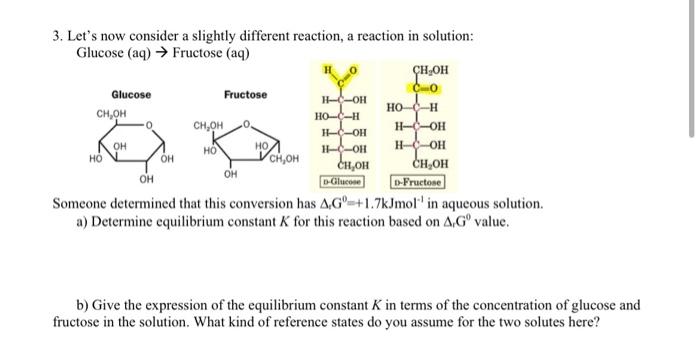
H-C-OH 3. Let's now consider a slightly different reaction, a reaction in solution: Glucose (aq) Fructose (aq) CHOH Glucose Fructose 0.0 Ho CH,OH HC I .. I-OH --OH 11-OH H-OH CH, OH CH OH OH D-Glucose D-Fructose Someone determined that this conversion has 4 G=+1.7kJmol' in aqueous solution. a) Determine equilibrium constant K for this reaction based on AG value. OH HO HO OH CH,OH OH b) Give the expression of the equilibrium constant K in terms of the concentration of glucose and fructose in the solution. What kind of reference states do you assume for the two solutes here 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


