Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help me please, i need to fill the mass balance table, and i also need to have all the steps required to fill the table
Help me please, i need to fill the mass balance table, and i also need to have all the steps required to fill the table in every stream. Help! Please but for today. I upvote.
Due to local demand needs, a project to build an acetic anhydride production plant is to be carried out. This process is carried out by cracking acetone to ketene and subsequently reacting it with acetic acid to produce the anhydride. The process begins in the cracking reactor (Reactor1), where acetone (a mixture of recycled acetone from the acetone column (15) and fresh acetone fed externally), reacts to form ketene, the main reagent for obtaining acetic anhydride. In Reactor1, cracking of acetone is carried out according to the main reaction () which decomposes acetone into methane and ketene. On the other hand, since ketene is an unstable compound, its deterioration occurs according to reaction (7). CH3COCH3 CH4 + CHCO (1) Acetone Methane + Ketene 2 CHCO CH4 + 2CO (7) Ketene >> Ethylene + Carbon Monoxide two In this Reactor1 there is a conversion of 61.944% of the total acetone fed, and 77.444% of the ketene. formed The products of the first reactor are sent to the next Reactor 2 (reactor-cooler/quench). This stream is contacted with a liquid stream of acetic acid. In said reactor, the reaction of acetic anhydride formation takes place from ketene and acetic acid (13). The operating conditions of this reactor are such that a total conversion of the ketene occurs. The acetic acid conversion in reactor 2 is 46.555%, and the ratios between streams 5 and 6 of the reactor output (N) are 4.527 for acetone, 4.097 for acetic acid and 0.0101 for acetic anhydride. The reactor outlet stream 6 contains only acetone, acetic acid and acetic anhydride.
CH3COOH + CHCO (CHCO) 0* Acetic acid + Ketene Acetic anhydride (13) *[C4H603] 0 AC0 The species that participate in the process are: Acetone (CH3COCH3), methane (CH4), carbon monoxide (CO), ketene (CH2CO), acetic acid (CH3COOH), acetic anhydride ((CH3CO)20) and ethylene (C2H4). The feed streams acetone (1) and acetic acid (9) are available as liquids and consider them like cigars. The figure shows the process flow diagram. In order to recover the current 5 from the top of Reactor2 is reactive, it feeds a partial condenser. From this, stream 7 leaves which is fed to an acetone absorption column and stream 8 which feeds the acetone column, which contains acetone, acetic acid and acetic anhydride. In this absorption column, stream 7 is contacted with a liquid stream 16 composed mainly of acetic acid (acetic acid is used in this case as acetone absorbent). The gaseous product obtained at the top of the absorber (10) is composed of acetone, CH4, CO, acetic acid, acetic anhydride and ethylene. Stream 10 leaving the absorption column goes to an off-process unit to recover acetic acid.
Stream 6 from the bottom of Reactor2 is sent to the acetic anhydride column where this product is separated from the rest of the components. The product is obtained at the bottom of the column. Stream 13 from the bottom of the acetic anhydride column would be the one containing the desired acetic anhydride product, with a purity of 99%. Stream 12 from the top of the anhydride column contains 95.61% of the acetic acid that enters the column and 0.997% of the anhydride entering this column. Stream 12 from the top of the acetic anhydride column is mixed with the two liquid streams (partial condenser outlet 8 and absorber bottom 11) and new stream 14 is fed to the acetone column. This mixture (stream 14) contains mainly acetone and acid, although there is also CH4, carbon monoxide, acetic anhydride and ethylene. Stream 14 is sent to the acetone column where acetic acid and acetone will be separated. Stream 15 from the top of this acetone-rich column is recycled to the process feed and contains, in addition to acetone, minor amounts of CH4, CO, acetic acid, acetic anhydride and ethylene. Stream 16 leaving the bottom of this column is rich in acetic acid and also contains acetone and acetic anhydride. This stream is recycled to the inlet of the acetone absorber. In the text of the problem and the Material Balance Table, there is all the information required to solve the problem. You are asked to complete the Material Balance Table and calculate the rates of the three reactions involved in the process.
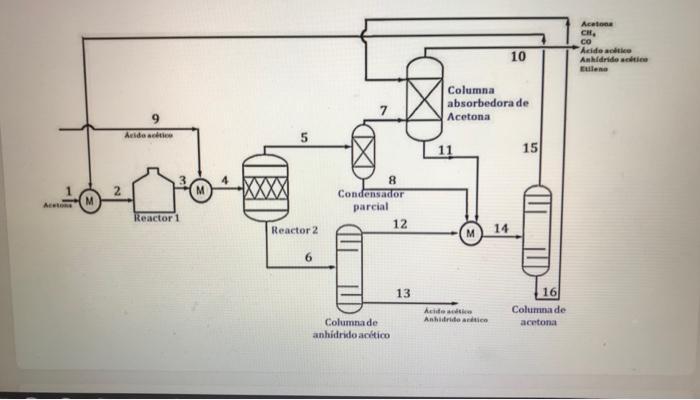
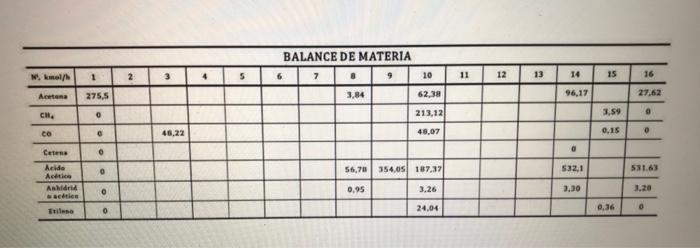
Aca CH, co Aicide atico Anhidrido soli Eulene 10 Columna absorbedora de Acetona Arts | 15 8 Condensador parcial 12 Reactor 1 Reactor 2 14 6 13 Acido Anbidatie 16 Columna de acetona Columnade anhidrido actico BALANCE DE MATERIA W. Imeld 1 2 3 5 7 . 9 10 11 12 13 14 15 16 Atena 275.5 3,84 62.38 96,17 27.62 CH, 0 213,12 3.59 46,22 48,07 0,15 . Celess . 0 56,70 354.05 187,37 532.1 531.63 heide Actie Anh acties 0.95 3.26 3.30 Etile 0 24,04 0 Aca CH, co Aicide atico Anhidrido soli Eulene 10 Columna absorbedora de Acetona Arts | 15 8 Condensador parcial 12 Reactor 1 Reactor 2 14 6 13 Acido Anbidatie 16 Columna de acetona Columnade anhidrido actico BALANCE DE MATERIA W. Imeld 1 2 3 5 7 . 9 10 11 12 13 14 15 16 Atena 275.5 3,84 62.38 96,17 27.62 CH, 0 213,12 3.59 46,22 48,07 0,15 . Celess . 0 56,70 354.05 187,37 532.1 531.63 heide Actie Anh acties 0.95 3.26 3.30 Etile 0 24,04 0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


