Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me solve with step by step explanation 7. Part 3: What is the general rate law for the given reaction? 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(n a) Rate=k[ClO2][OH] b)
help me solve with step by step explanation
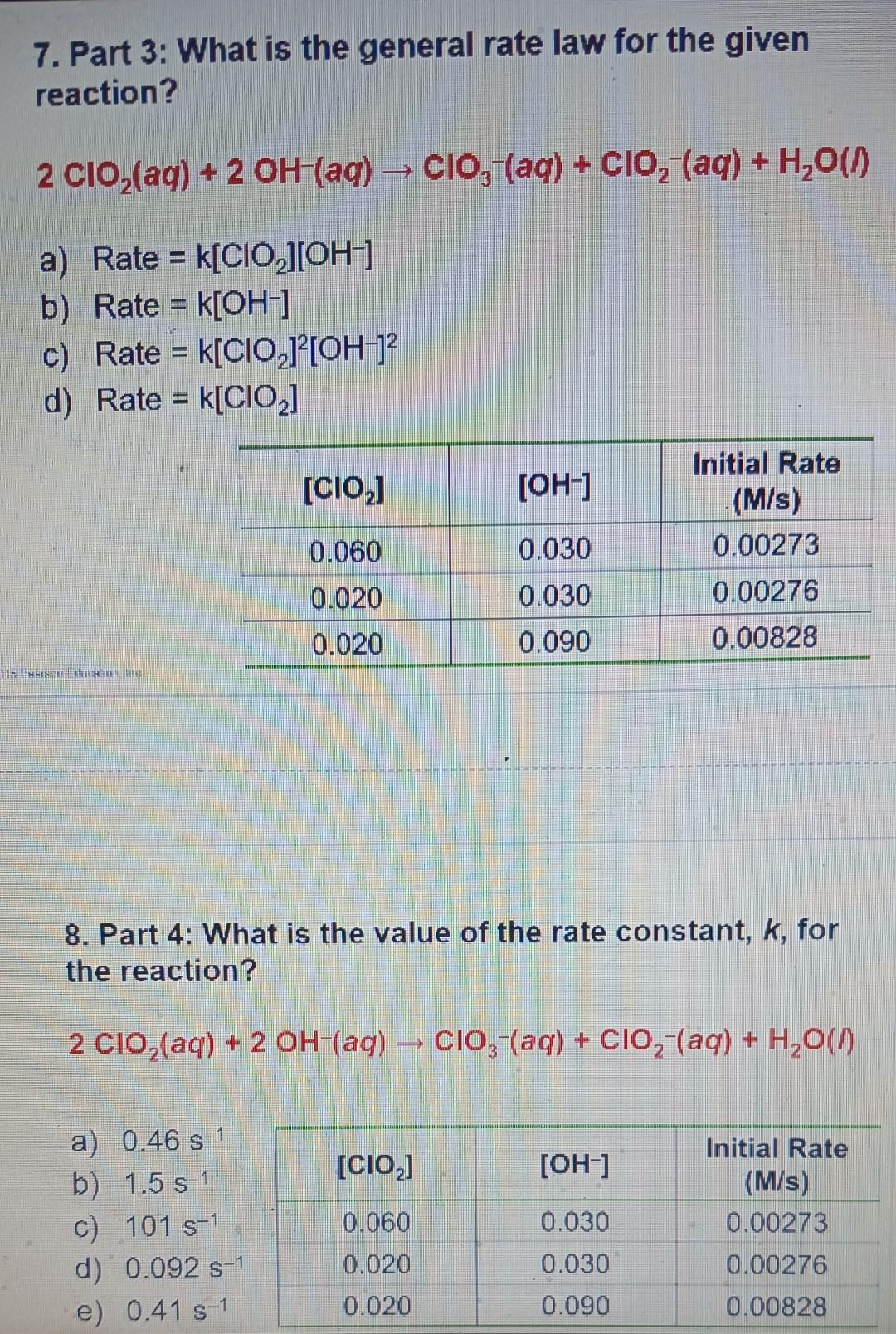
7. Part 3: What is the general rate law for the given reaction? 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(n a) Rate=k[ClO2][OH] b) Rate =k[OH] c) Rate =k[ClO2]2[OH]2 d) Rate =k[ClO2] 8. Part 4: What is the value of the rate constant, k, for the reaction? 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(I) a) 0.46s1 b) 1.5s1 c) 101s1 d) 0.092s1 e) 0.41s1 \begin{tabular}{|c|c|c|} \hline[ClO2] & {[OH]} & InitialRate(M/s) \\ \hline 0.060 & 0.030 & 0.00273 \\ \hline 0.020 & 0.030 & 0.00276 \\ \hline 0.020 & 0.090 & 0.00828 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


