Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me to solve this problem, thank you. Liquid reaction A2B is taking place in a steady state packed bed reactor that has total available
help me to solve this problem, thank you. 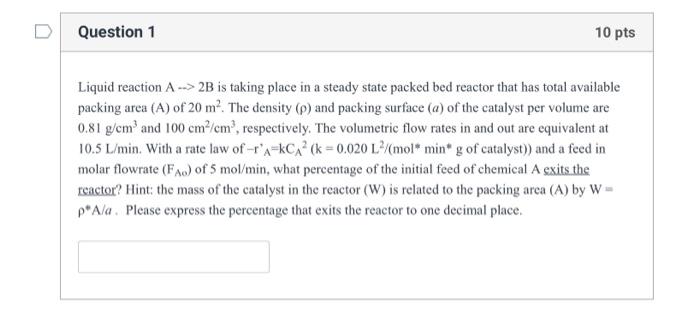
Liquid reaction A2B is taking place in a steady state packed bed reactor that has total available packing area (A) of 20m2. The density () and packing surface (a) of the catalyst per volume are 0.81g/cm3 and 100cm2/cm3, respectively. The volumetric flow rates in and out are equivalent at molar flowrate (FAo) of 5mol/min, what percentage of the initial feed of chemical A exits the reactor? Hint: the mass of the catalyst in the reactor (W) is related to the packing area (A) by W= A/a. Please express the percentage that exits the reactor to one decimal place 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


