Question: Help needed for part C. CHEM 162L Experiment 4 Page 1 LeChatelier's Principle See section 13.7 of the textbook for background information. Objectives: To learn



 Help needed for part C.
Help needed for part C.

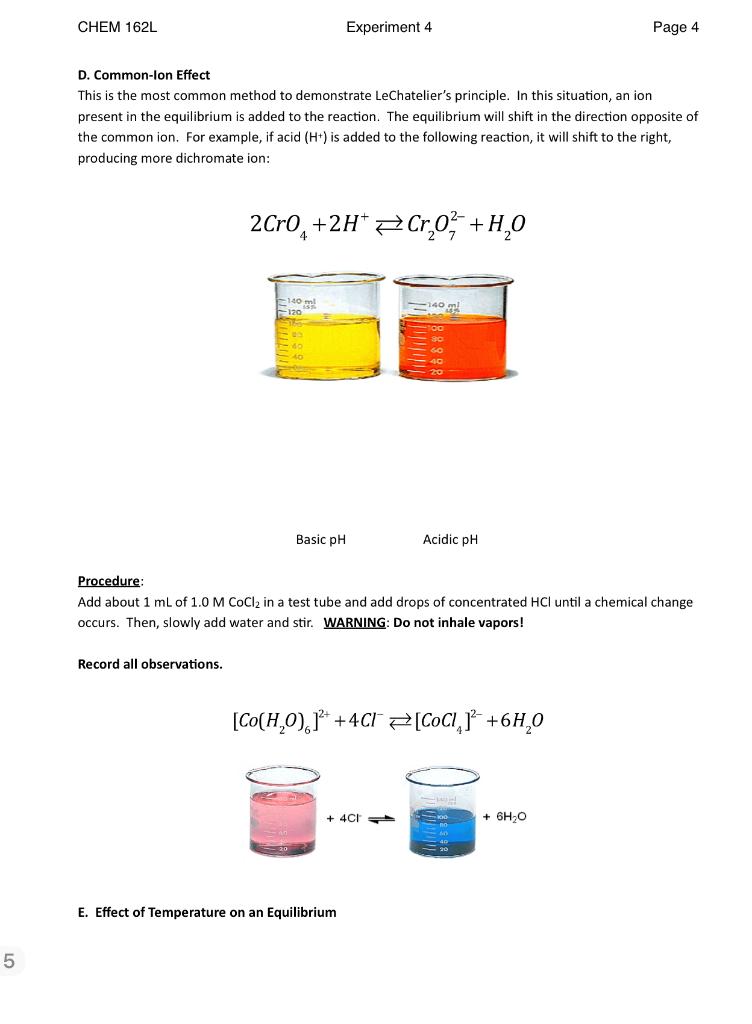
CHEM 162L Experiment 4 Page 1 LeChatelier's Principle See section 13.7 of the textbook for background information. Objectives: To learn how chemical equilibria are influenced by a change in concentration of reactant or product. To learn how temperature affects a chemical equilibrium. Experiment: A. How ammonia will impact a chemical equilibria containing a metal ion. Ammonia forms complexes with many metal ions. Upon doing so, the color of the solution, which is due to the metal ion, changes. For example, copper (11) ion in solution has a sky-blue color. When copper (11) ion is complexed with ammonia, the complex has a deep-blue color. C * +4 NH, [Cu(NHJ) J Im01 Imo o [Cu(H,0)612 [Cu(NH3)4(H2O),12 What happens to the equilibria when HCl is added to the reaction? Hint: ammonia reacts with HCI according to the following equation: NH3(aq) + H+(aq) NH(aq) Procedure 1. Add 1 mL of 0.10 M CuSO4 to a small test tube. Add drops of concentrated NH3 until a color change occurs. WARNING: Do not inhale vapors! 2. Add drops of 1.0 M HCl until the color changes again. Record your observations and write equations showing what is happening. CHEM 162L Experiment 4 Page 2 B. Equilibria involving silver ion. Silver ion forms many different equilibrium complexes that can be readily observed. In the first part of this section, we will observe the effects of changing concentration of reactants on the equilibrium of the following reaction: 2 Ag+ +0 =Ag,CO2 (s) In this section, you will add reagents that will change the concentration of silver ion or carbonate anion according the following reactions: CO3 +2H+ H.CO Ag+ +C1" AgCI(S) In step #4 of the procedure, you will generate the following equilibrium from the original solution: Agt +2 NH, [Ag(NH2)21* This reaction occurs when NH3 is added to the silver/carbonate equilibrium. Subsequent reagents, when added to the reaction, will cause a change in the equilibrium according to the following reactions: Ag + Agl(s) 2Ag* +S? Ag,S(s) Ag A92CO3 Ag(NH3)2* Chemical Reactions of Ag* Agci Ag(S203)23- Agi Ag2S CHEM 162L Experiment 4 Page 3 Procedure: WARNING: USE CARE WITH AgNO3!! IT CAN STAIN SKIN! Add 1 mL of 0.01 M AgNO3 to 1 mL of 0.1 M Na2CO3 and observe what happens. 2. Add drops of 6 M HNO: (a source of H+) until a chemical change occurs. 3. Add 5 drops of 0.1 M HCI. 4. Add concentrated NH3 until a chemical change occurs. (WARNING: Do not inhale vapors!) 5. Add drops of 6 M HNO3 until a chemical change occurs. 6. Add concentrated NH3 until a chemical change occurs. 7. Add drops of 0.1 M kl until a chemical change occurs. 8. Add drops of 0.1 M NazS until a chemical change occurs. Record all observations and use the equations above to write an equation showing what is occurring in each step. C. Buffers Buffers are substances that resist change in pH because they are composed of a weak acid, which will neutralize added base, and weak base, which will neutralize an added acid. The buffer you will explore is acetic acid / acetate: CH COOH CH COO+H+ added base: CH,COOH +OH CH COO +H2O added acid: CH,C00 +H* CH,COOH Procedure 1. Add 10 drops of 0.10 M acetic acid to two medium test tubes. 2. Add 3 drops of universal indicator. Estimate the pH using color scale. 3. Add 10 drops of 0.10 M sodium acetate to each well. Estimate pH. 4. To two new test tubes, add -20 drops of deionized water and 3 drops of universal indicator and estimate pH. 5. Add 5 drops of 0.10 M HCl to one test tube containing buffer and to one test containing water. Estimate the change in pH for each solution. 6. Add 5 drops of 0.10 M NaOH to the remaining test tube containing buffer and to the test tube containing deionized water. Estimate the change in pH for each solution. Record all observations and pH values in your notebook. On the report sheet, record the pH values. CHEM 162L Experiment 4 Page 4 D. Common-lon Effect This is the most common method to demonstrate LeChatelier's principle. In this situation, an ion present in the equilibrium is added to the reaction. The equilibrium will shift in the direction opposite of the common ion. For example, if acid (H+) is added to the following reaction, it will shift to the right, producing more dichromate ion: 2Cro, +2H* Cro? +H20 140 ml 120 140 Basic pH Acidic pH Procedure: Add about 1 mL of 1.0 M CoCl2 in a test tube and add drops of concentrated HCl until a chemical change occurs. Then, slowly add water and stir. WARNING: Do not inhale vapors! Record all observations. [Co(H,0), 14+ +4C1 =[CoCl,]? +6H40 + 4C + 6H2O E. Effect of Temperature on an Equilibrium 5 CHEM 162L Experiment 4 Page 5 A change in temperature will change the value of the equilibrium constant causing the reaction to proceed in one direction or the other. In this part of the experiment you will determine the effect of temperature on the following equilibrium: [Co(H20), 12+ + 4C1 = [COCI, 1*- +6H40 Procedure: Add about 1 mL of 1.0 M CoCl2 in to a test tube and place into a boiling water bath. Observe the chemical change that occurs. Exp. 4: LeChatelier's Principle Report Sheet A. Write an equation(s) to explain the color changes observed. Cu2+ + 4NH3 [Cu(NH3)4] 2+ CUSO4 + Unte. 27 (SKY BLUE) (Deep Blue) B. Write an equation to explain each step. 1. [CU (NH3)4] 2t + 5042 > 2AgNONO,008 Ag2003 + 2NANO, 2. Ag 2004+ HNO3 + H20+002+AgNO, CO2 3. AgNO3 + HCl AgCl (s) + HNO3 4. Ag2+ + 2NH2 = (A g(NH3)2]* s [Ag (Nitz)2 ]*+ 2H003 2NH4NO3 + Agt + 5. 6. Agt + NH3 = [Ag(NH3)2]" 7. Agtt12AgI(s) 7 8 Name ... 2 Ag+ + Nazwa Ag, St Nat Lab Day/Time C. Record the approximate pH values of the following: Water 1 2 Initial pH With HCI Change in pH With NaOH Change in pH Buffer 1 2 Acetic acid only With sodium acetate With HCI Change in pH With NaOH Change in pH Comment on the differences observed in the water versus the buffer. D. Observations: E. What effect does heat have on the equilibrium? CHEM 162L Experiment 4 Page 1 LeChatelier's Principle See section 13.7 of the textbook for background information. Objectives: To learn how chemical equilibria are influenced by a change in concentration of reactant or product. To learn how temperature affects a chemical equilibrium. Experiment: A. How ammonia will impact a chemical equilibria containing a metal ion. Ammonia forms complexes with many metal ions. Upon doing so, the color of the solution, which is due to the metal ion, changes. For example, copper (11) ion in solution has a sky-blue color. When copper (11) ion is complexed with ammonia, the complex has a deep-blue color. C * +4 NH, [Cu(NHJ) J Im01 Imo o [Cu(H,0)612 [Cu(NH3)4(H2O),12 What happens to the equilibria when HCl is added to the reaction? Hint: ammonia reacts with HCI according to the following equation: NH3(aq) + H+(aq) NH(aq) Procedure 1. Add 1 mL of 0.10 M CuSO4 to a small test tube. Add drops of concentrated NH3 until a color change occurs. WARNING: Do not inhale vapors! 2. Add drops of 1.0 M HCl until the color changes again. Record your observations and write equations showing what is happening. CHEM 162L Experiment 4 Page 2 B. Equilibria involving silver ion. Silver ion forms many different equilibrium complexes that can be readily observed. In the first part of this section, we will observe the effects of changing concentration of reactants on the equilibrium of the following reaction: 2 Ag+ +0 =Ag,CO2 (s) In this section, you will add reagents that will change the concentration of silver ion or carbonate anion according the following reactions: CO3 +2H+ H.CO Ag+ +C1" AgCI(S) In step #4 of the procedure, you will generate the following equilibrium from the original solution: Agt +2 NH, [Ag(NH2)21* This reaction occurs when NH3 is added to the silver/carbonate equilibrium. Subsequent reagents, when added to the reaction, will cause a change in the equilibrium according to the following reactions: Ag + Agl(s) 2Ag* +S? Ag,S(s) Ag A92CO3 Ag(NH3)2* Chemical Reactions of Ag* Agci Ag(S203)23- Agi Ag2S CHEM 162L Experiment 4 Page 3 Procedure: WARNING: USE CARE WITH AgNO3!! IT CAN STAIN SKIN! Add 1 mL of 0.01 M AgNO3 to 1 mL of 0.1 M Na2CO3 and observe what happens. 2. Add drops of 6 M HNO: (a source of H+) until a chemical change occurs. 3. Add 5 drops of 0.1 M HCI. 4. Add concentrated NH3 until a chemical change occurs. (WARNING: Do not inhale vapors!) 5. Add drops of 6 M HNO3 until a chemical change occurs. 6. Add concentrated NH3 until a chemical change occurs. 7. Add drops of 0.1 M kl until a chemical change occurs. 8. Add drops of 0.1 M NazS until a chemical change occurs. Record all observations and use the equations above to write an equation showing what is occurring in each step. C. Buffers Buffers are substances that resist change in pH because they are composed of a weak acid, which will neutralize added base, and weak base, which will neutralize an added acid. The buffer you will explore is acetic acid / acetate: CH COOH CH COO+H+ added base: CH,COOH +OH CH COO +H2O added acid: CH,C00 +H* CH,COOH Procedure 1. Add 10 drops of 0.10 M acetic acid to two medium test tubes. 2. Add 3 drops of universal indicator. Estimate the pH using color scale. 3. Add 10 drops of 0.10 M sodium acetate to each well. Estimate pH. 4. To two new test tubes, add -20 drops of deionized water and 3 drops of universal indicator and estimate pH. 5. Add 5 drops of 0.10 M HCl to one test tube containing buffer and to one test containing water. Estimate the change in pH for each solution. 6. Add 5 drops of 0.10 M NaOH to the remaining test tube containing buffer and to the test tube containing deionized water. Estimate the change in pH for each solution. Record all observations and pH values in your notebook. On the report sheet, record the pH values. CHEM 162L Experiment 4 Page 4 D. Common-lon Effect This is the most common method to demonstrate LeChatelier's principle. In this situation, an ion present in the equilibrium is added to the reaction. The equilibrium will shift in the direction opposite of the common ion. For example, if acid (H+) is added to the following reaction, it will shift to the right, producing more dichromate ion: 2Cro, +2H* Cro? +H20 140 ml 120 140 Basic pH Acidic pH Procedure: Add about 1 mL of 1.0 M CoCl2 in a test tube and add drops of concentrated HCl until a chemical change occurs. Then, slowly add water and stir. WARNING: Do not inhale vapors! Record all observations. [Co(H,0), 14+ +4C1 =[CoCl,]? +6H40 + 4C + 6H2O E. Effect of Temperature on an Equilibrium 5 CHEM 162L Experiment 4 Page 5 A change in temperature will change the value of the equilibrium constant causing the reaction to proceed in one direction or the other. In this part of the experiment you will determine the effect of temperature on the following equilibrium: [Co(H20), 12+ + 4C1 = [COCI, 1*- +6H40 Procedure: Add about 1 mL of 1.0 M CoCl2 in to a test tube and place into a boiling water bath. Observe the chemical change that occurs. Exp. 4: LeChatelier's Principle Report Sheet A. Write an equation(s) to explain the color changes observed. Cu2+ + 4NH3 [Cu(NH3)4] 2+ CUSO4 + Unte. 27 (SKY BLUE) (Deep Blue) B. Write an equation to explain each step. 1. [CU (NH3)4] 2t + 5042 > 2AgNONO,008 Ag2003 + 2NANO, 2. Ag 2004+ HNO3 + H20+002+AgNO, CO2 3. AgNO3 + HCl AgCl (s) + HNO3 4. Ag2+ + 2NH2 = (A g(NH3)2]* s [Ag (Nitz)2 ]*+ 2H003 2NH4NO3 + Agt + 5. 6. Agt + NH3 = [Ag(NH3)2]" 7. Agtt12AgI(s) 7 8 Name ... 2 Ag+ + Nazwa Ag, St Nat Lab Day/Time C. Record the approximate pH values of the following: Water 1 2 Initial pH With HCI Change in pH With NaOH Change in pH Buffer 1 2 Acetic acid only With sodium acetate With HCI Change in pH With NaOH Change in pH Comment on the differences observed in the water versus the buffer. D. Observations: E. What effect does heat have on the equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


