Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help NO(s) and O2(s) react as follows: 2NO(s)+O2(s)2NO2(s) It has been determined experimentally that the rate law of the above reaction is second-order in NO
Help 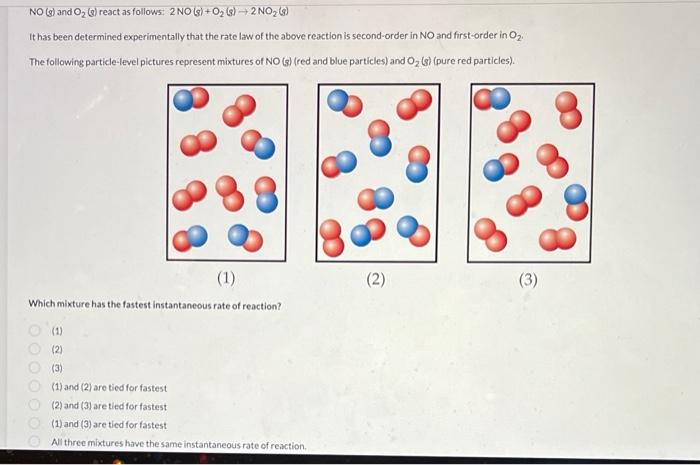
NO(s) and O2(s) react as follows: 2NO(s)+O2(s)2NO2(s) It has been determined experimentally that the rate law of the above reaction is second-order in NO and first-order in O2 : The following particle-level pictures represent mixtures of NO(s) (red and blue particles) and O2 (s) (pure red particles). (1) (2) (3) Which mixture has the fastest instantaneous rate of reaction? (1) (2) (3) (1) and (2) are tied for fastest (2) and (3) are tied for fastest (1) and (3) are tied for fastest All three mixtures have the same instantaneous rate of reiction 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


