help on all plz

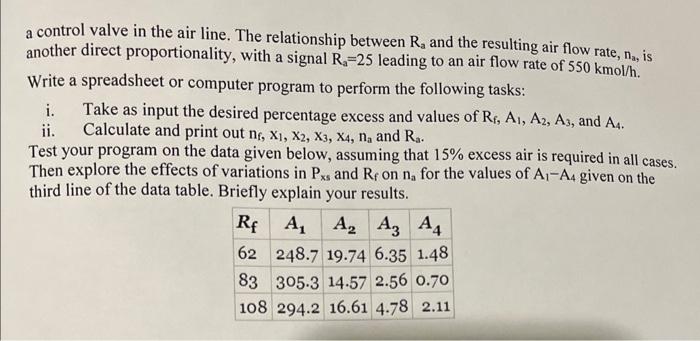
Natural gas containing a mixture of methane, ethane, propane, and butane is burned in a furnace with excess air. See Example 4.8-3 for help getting started. a) One hundred kmol/h of a gas containing 94.4 mole\% methane, 3.40% ethane, 0.60% propane, and 0.50% butane is to be burned with 17% excess air. Calculate the required molar flow rate of the air. b) Given the following terminology, derive an expression for na in terms of the other variables. Check your formula with the results of Part a). n1(kmol/h)=molarflowofthefuelgasx1,x2,x3,x4=molefractionsofmethane,ethane,propane,andbutane,respectively,inthefuelPxs=percentexcessairna(kmol/h)=molarflowrateoftheairfedtothefurnace c) Suppose the feed rate and composition of the fuel gas are subject to periodic variations, and a process control computer is to be used to adjust the flow rate of air to maintain a constant percentage excess. A calibrated electronic flowmeter in the fuel gas line transmits a signal Rf that is directly proportional to the flow rate (n=Rf), with a flow rate of 75.0kmol/h yielding a signal Rf=60. The fuel gas composition is obtained with an on-line gas chromatograph. A sample of the gas is injected into the gas chromatograph (GC), and signals A1,A2,A3, and A4, which are directly proportional to the moles of methane, ethane, propane, and butane, respectively, in the sample, are transmitted. (Assume the same proportionality constant for all species.) The control computer processes these data to determine the required air flow rate and then sends a signal Ra to a control valve in the air line. The relationship between Ra and the resulting air flow rate, na, is another direct proportionality, with a signal Ra=25 leading to an air flow rate of 550kmol/h. Write a spreadsheet or computer program to perform the following task: i. Take as input the desired percentage excess and values of Rf,A1,A2,A3, and A4. ii. Calculate and print out nf,x1,x2,x3,x4,na and Ra. Test your program on the data given below, assuming that 15% excess air is required in all cases. Then explore the effects of variations in Pxs and Rf on na for the values of A1A4 given on the third line of the data table. Briefly explain your results