Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help on these two asap. first pic is question 1 and pics 2&3 are question 2 The structure below has an unusually large dipole. Draw
help on these two asap. first pic is question 1 and pics 2&3 are question 2 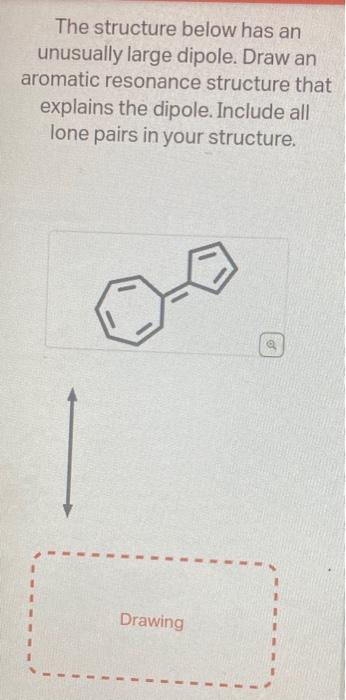
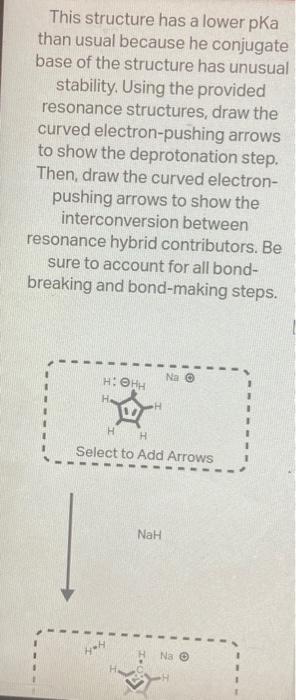
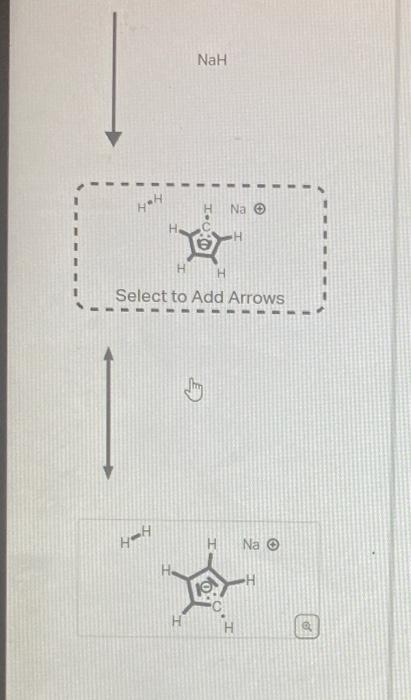
The structure below has an unusually large dipole. Draw an aromatic resonance structure that explains the dipole. Include all lone pairs in your structure. This structure has a lower pKa than usual because he conjugate base of the structure has unusual stability. Using the provided resonance structures, draw the curved electron-pushing arrows to show the deprotonation step. Then, draw the curved electronpushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bondbreaking and bond-making steps. NaH Select to Add Arrows 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


