Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. Identify cach compound as Empirical or Molecular. Write your answer on the space provided. 1. Olucose (Ch:0) 2. Hydmgen Peroxdde (H2O2) 3. Dichlorine

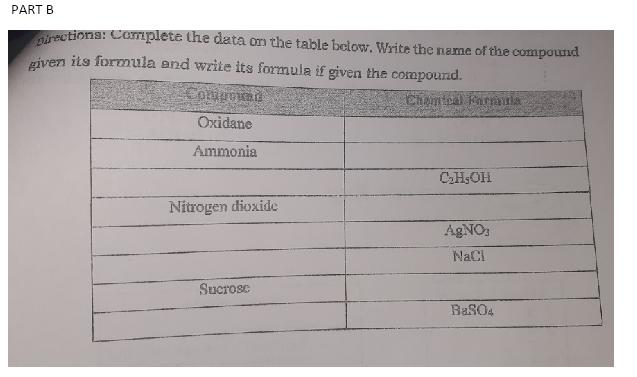

A. Identify cach compound as Empirical or Molecular. Write your answer on the space provided. 1. Olucose (Ch:0) 2. Hydmgen Peroxdde (H2O2) 3. Dichlorine Hexoxide (ClaO) 4. Buune (C.H10) 5. Fructose (CSH12O6) 6. Methyl Acetate (Call,Oa) 7. Ammonia (NH) 8. Barium Chloride (BaCla) 9. Lactic Acid (CaH6Oa) 10. Naphthalene (CsH4) PART B irections: Complete the data on the table below. Write the name of the compound given its formula and write its formula if given the compound. Componad Oxidane Ammonia CHsOH Nitrogen dioxide AgNO NaCl Sucrosc BaS04 PART C Directions: Write the letter of the correct answer on a separate sheet of paper. 1. Which of the following shows an example of Empirical formula? c. CjaHza01 d. NaCi 2. Which of the following shows an example of Empirical formula? c. ClaOs d. CoH1206 3. Which of the following shows an example of Molecular formula? c. CO3 d. NaCI 4. Which of the following shows an example of Molecular formula? c. NaCl d. ClLOG 5. Which of the following shows an example of Structural formula? c. Na:CI d. H=Cl a. CaH1ON4O2 b.H2O2 a. MgsN2 b. HaO a. PO4 b. H2O2 a. PO4 b.CO. a. Ca*O b. C/H/O 6. Which of the following gives the simplest ration of atoms in a molecule? c. molecular formula d. terrestrial formula 7. Which of the following gives the exact number of each atom in a molecule? C. molecular formula d. terrestrial formula 8. What is the empirical formula if you have 35.98% aluminum and 64.02% a. empirical formula b.chemical formula a. empirical formula b. chemical formula sulfur? a. AIS b. AIS2 C. Al2S d. A12S3 the empirical formula if you have 36.84% nitrogen and 63. 16% a. NO b. N204 he following: 42.07% Na, 18.89% P. and 39.04% O, determine the mula. . NAPO2 - NA3PO4 C. N203 d. N205 c. NA2PO3 d. NAPO4
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Answer Part1 Empirical formulas show the simplest wholenumber ratio o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


