Question
Which intermolecular force(s) do the following pairs of molecules experience? Pentane 1st attempt Part 1 (1 point) pentane and pentanol Choose one or more:
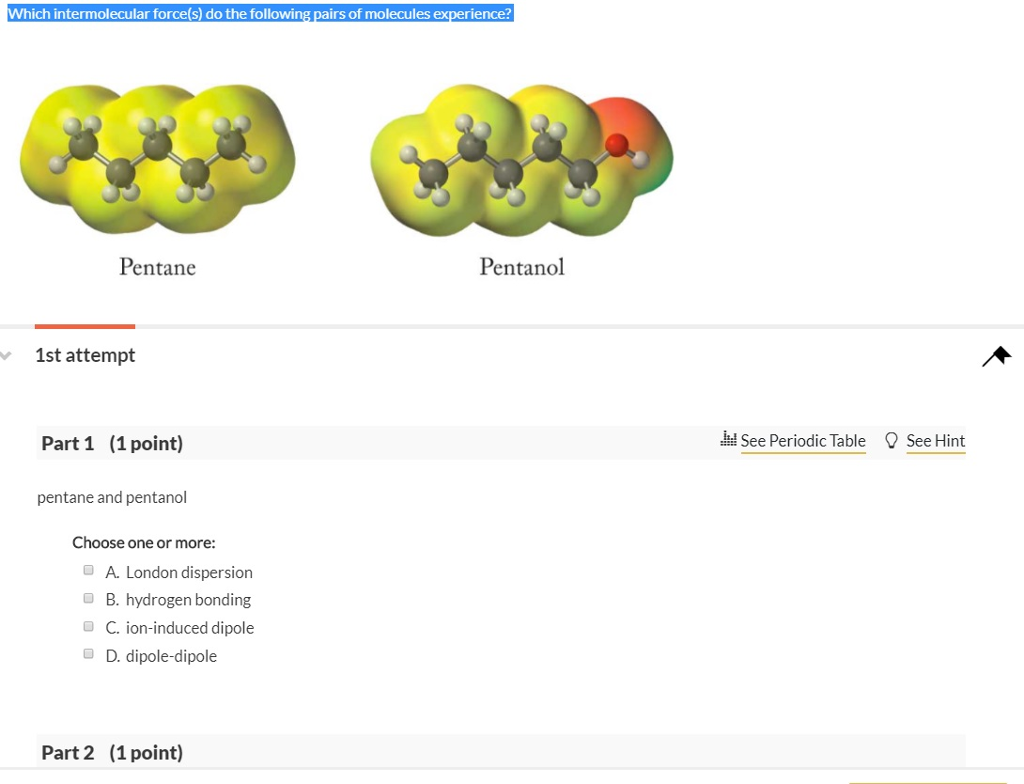

Which intermolecular force(s) do the following pairs of molecules experience? Pentane 1st attempt Part 1 (1 point) pentane and pentanol Choose one or more: A. London dispersion B. hydrogen bonding C. ion-induced dipole D. dipole-dipole Part 2 (1 point) Pentanol See Periodic Table See Hint Part 2 (1 point) pentanol with another molecule of pentanol Choose one or more: A. London dispersion B. dipole-dipole C. hydrogen bonding D. ion-induced dipole Part 3 (1 point) pentane with another molecule of pentane Choose one or more: A. London dispersion B. dipole-dipole C. hydrogen bonding D. ion-induced dipole
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Part 1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Linear Algebra
Authors: Peter J. Olver, Cheri Shakiban
1st edition
131473824, 978-0131473829
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



